- Ingredients
blends tailored to your unique needs.

RECLAIM YOUR ATTENTION
Starter Kits Purchased
Ingredients Tested
Find a Blend that Works
10,000+ Verified 5-star Reviews
Choose your thesis blend
Get started for just $79/month
Maintain focus and Support attention
Fight fatigue and Build mental stamina
Maintain willpower and Support productivity
Support memory and Promote deep thinking
Verbal fluency and Maintain confidence
Feelings of calm and Positive outlook
- Promote Energy
- Fight Fatigue
- Build Mental Stamina
TeaCrine® has been shown to increase energy, motivation, and concentration.
- Maintain Focus
- Support Attention
- Enter Flow State
Alpha-GPC has been shown to support healthy cognitive function and physical performance.
- Maintain Willpower
- Manage Procrastination
- Support Productivity
CDT (Dynamine® ) has been shown to help with cognitive control and reaction time during mentally demanding tasks.
- Support Memory
- Promote Deep Thinking
- Maintain Processing Speed
Synapsa® has been shown to significantly improve working memory.
- Spark Inspiration
- Support Verbal Fluency
- Manage Stress
KSM-66® Ashwagandha has been shown to significantly reduce stress and anxiety.
- Encourage calm state
- Support mental flexibility
- Promote positive outloook
Zembrin® has been shown to improve in mood and disposition.
- GMP Certified
- Gluten Free
Nootropics aren’t one size fits all
We formulate potent nutrient compounds to enhance mental performance and make personalized recommendations based on your goals and unique brain chemistry
Your Personalized Thesis Journey
1. take the quiz.
Tell us about yourself and your goals. We’ll use your answers to determine your baseline and build your recommendations.
2. Get Your Starter Kit
You’ll sample 4 blends over the course of the month to understand what you can accomplish with each formulation.
3. Optimize with a Coach
Check in with your wellness coach and our nootropics experts to customise your blends based on your experience so far.
A powerful, natural way to boost your cognition
Thesis can help you.
- Raise your energy level
- Feel more motivated
- Clear brain fog
- Increase focus
We’ve Helped Over 500,000 People Find Their Formula
“Motivation gets me going during my six days in a row at work, and Clarity keeps my mind sharp and alert so I’m performing at my best . Creativity does just as it says -- I love this one for when I’m doing the Reading Comprehension and/or Arguments section of LSAT prep; I truly feel like it gives me an edge.”
Britney’S BLENDS
"I did feel different since day one. I got more motivated and had an overall better mood. [Thesis] has been a game changer for me."
Ondrej's BLENDS
“I always feel energized and focus without the afternoon crash that I typically experience with coffee. I can get hours of work done and stay motivated all day.”
Renee’S BLENDS
"For someone who has struggled with attention and staying awake, [Thesis has] been life saving."
Trish's BLENDS
Trusted by experts
Dr. gabrielle lyon.
Functional Medicine & Nutritional Sciences
“I work with CEOs, celebrities, and other top performers in my practice. Thesis is what I recommend and take personally for focus and cognition. I even used it to help me nail my first TedX talk.”
Gabrielle’s blends
Professional Basketball Player & Mental Health Advocate
“With a busy life on and off the court, Thesis gives me energy and focus to get through the longest days and keep me sharp.”
Kevin's blends
Ultra-endurance Athlete & Nutrition Advocate
"Thesis has provided a substantial benefit to my ability to focus. Creativity works best for me — I take it 30 mins before a podcast or writing and it helps get me into the zone."
RICH’s blends
Pure and effective ingredients
Potent active ingredients.
Quality counts when supplementing, and only the active ingredients in a blend make an impact.
Clinically Studied Dosage
We only use nutrients that have been proven to safely deliver desired effects in clinical trials.
All ingredients in each batch are tested with a third party lab to ensure optimal potency and purity.
“ The Thesis process was developed by systematically testing different combinations of high quality ingredients. We made the process of finding the right nootropics quicker & safer.”

DAN FREED CEO & Founder, Thesis
Our research and product development teams review clinical studies and information on safety, side effects, and any potential interactions for each ingredient being considered for a Thesis blend.
Each ingredient goes through two rounds of internal testing, in which members of our research and product development team share feedback on individual ingredients.
The research and product development team reviews existing clinical literature about synergistic benefits between ingredients and integrates it as we continue to formulate, developing 2-4 blends to move forward to Phase 3 testing.
We test each prospective blend internally, as members of our research and product development team try each of the blends before we move forward to Beta testing.
Next, we test multiple iterations of each blend with a group of 100 Thesis beta group customers and collect quantitative and qualitative feedback to help us refine the final blend.
We finalize our winning blend (based on the Alpha and Beta test feedback) by completing a final round of safety testing by our third party lab partners before we release it. Ongoing safety testing occurs with each batch of production.
After the blend undergoes third party lab testing for safety, we launch a limited release to 5,000 customers to help us gather additional feedback and work through our supply chain process
Once a blend passes the limited release phase, we launch full production in a cGMP facility and release it to all customers.
The Thesis Story
As long as I can remember, people thought I was stupid, lazy, or unmotivated. I started to believe it. In school, I would read the same page over and over again, without absorbing anything. At 16, I dropped out of high school and went to work at a sandwich shop.
Fast-forward ten years — I scored in the 99th percentile on the GMAT and earned Master’s degrees from Yale and INSEAD. Nootropics turned everything around for me, and helped me form the positive habits that I built my success on. Once I balanced my brain chemistry, I could perform like never before.
I take Energy to get me going in the morning and Motivation to power through long afternoons.
DAN’s blends
Benefits and Harms of ‘Smart Drugs’ (Nootropics) in Healthy Individuals
- Review Article
- Published: 02 April 2022
- Volume 82 , pages 633–647, ( 2022 )
Cite this article

- Fabrizio Schifano 1 ,
- Valeria Catalani 1 ,
- Safia Sharif 1 ,
- Flavia Napoletano 2 ,
- John Martin Corkery 1 ,
- Davide Arillotta 1 ,
- Suzanne Fergus 1 ,
- Alessandro Vento 3 , 4 , 5 &
- Amira Guirguis 1 , 6
6123 Accesses
16 Citations
17 Altmetric
Explore all metrics
A Correction to this article was published on 27 April 2022
This article has been updated
‘Smart drugs’ (also known as ‘nootropics’ and ‘cognitive enhancers’ [CEs]) are being used by healthy subjects (i.e. students and workers) typically to improve memory, attention, learning, executive functions and vigilance, hence the reference to a ‘pharmaceutical cognitive doping behaviour’. While the efficacy of known CEs in individuals with memory or learning deficits is well known, their effect on non-impaired brains is still to be fully assessed. This paper aims to provide an overview on the prevalence of use; putative neuroenhancement benefits and possible harms relating to the intake of the most popular CEs (e.g. amphetamine-type stimulants, methylphenidate, donepezil, selegiline, modafinil, piracetam, benzodiazepine inverse agonists, and unifiram analogues) in healthy individuals. CEs are generally perceived by the users as effective, with related enthusiastic anecdotal reports; however, their efficacy in healthy individuals is uncertain and any reported improvement temporary. Conversely, since most CEs are stimulants, the related modulation of central noradrenaline, glutamate, and dopamine levels may lead to cardiovascular, neurological and psychopathological complications. Furthermore, use of CEs can be associated with paradoxical short- and long-term cognitive decline; decreased potential for plastic learning; and addictive behaviour. Finally, the non-medical use of any potent psychotropic raises serious ethical and legal issues, with nootropics having the potential to become a major public health concern. Further studies investigating CE-associated social, psychological, and biological outcomes are urgently needed to allow firm conclusions to be drawn on the appropriateness of CE use in healthy individuals.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Substances used and prevalence rates of pharmacological cognitive enhancement among healthy subjects
Is methylphenidate beneficial and safe in pharmacological cognitive enhancement.

Pharmacological Neuroenhancement: Substances and Epidemiology
Change history, 27 april 2022.
A Correction to this paper has been published: https://doi.org/10.1007/s40265-022-01716-0
Frati P, Kyriakou C, Del RA, Marinelli E, Vergallo GM, Zaami S, Busardò FP. Smart drugs and synthetic androgens for cognitive and physical enhancement: revolving doors of cosmetic neurology. Curr Neuropharmacol. 2015;13:5.
Article CAS PubMed PubMed Central Google Scholar
Bostrom N, Sandberg A. Cognitive enhancement: methods, ethics, regulatory challenges. Sci Eng Ethics. 2009;15:311–41.
Article PubMed Google Scholar
Sharif S, Guirguis A, Fergus S, Schifano F. The use and impact of cognitive enhancers among university students: a systematic review. Brain Sci. 2021;11(3):355.
Fan Y, Zhang Y, Li J, Liu Y, Chang H, Jiang Y, et al. Association between healthy eating index-2015 and various cognitive domains in US adults aged 60 years or older: the National Health and Nutrition Examination Survey (NHANES) 2011–2014. BMC Public Health. 2021;21:1–11.
Article Google Scholar
Hamidovic A, Dlugos A, Palmer AA, De Wit H. Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatr Genet. 2010;20:85.
Article PubMed PubMed Central Google Scholar
Turner TH, Rodriguez-Porcel F, Lee P, Teague K, Heidelberg L, Jenkins S, et al. Executive function and dopamine response in Parkinson’s disease freezing of gait. Parkinson Relat Disord. 2021;92:46–50.
Giurgea C. Pharmacology of integrative activity of the brain. Attempt at nootropic concept in psychopharmacology. Actual Pharmacol. 1972;25:115–56.
CAS Google Scholar
Napoletano F, Schifano F, Corkery J, Guirguis A, Arillotta D, Zangani C, et al. The psychonauts’ world of cognitive enhancers. Front Psychiatry. 2020;11:546796. https://doi.org/10.3389/FPSYT.2020.546796 .
Million Insights Nootropics market to register 12.5% CAGR by 2025 owing to rising awareness regarding mental illness, growing expenditure on healthcare & wellness. 2021. https://www.prnewswire.com/news-releases/nootropics-market-to-register-12-5-cagr-by-2025-owing-to-rising-awareness-regarding-mental-illness-growing-expenditure-on-healthcare--wellness--million-insights-301247932.html . Accessed 30 Sep 2021
Dresler M, Sandberg A, Bublitz C, Ohla K, Trenado C, Mroczko-Wąsowicz A, et al. Hacking the brain: dimensions of cognitive enhancement. ACS Chem Neurosci. 2018;10:1137–48.
Valentine G, Sofuoglu M. Cognitive effects of nicotine: recent progress. Curr Neuropharmacol. 2018;16(4):403–14. https://doi.org/10.2174/1570159X15666171103152136 .
Morelli M, Tognotti E. Brief history of the medical and non-medical use of amphetamine-like psychostimulants. Exp Neurol. 2021;342:113754.
Article CAS PubMed Google Scholar
Wade L, Forlini C, Racine E. Generating genius: how an Alzheimer’s drug became considered a “cognitive enhancer” for healthy individuals. BMC Med Ethics. 2014;15:37. https://doi.org/10.1186/1472-6939-15-37 .
Froestl W, Muhs A, Pfeifer A. Cognitive enhancers (Nootropics). Part 2: drugs interacting with enzymes. J Alzheimer’s Dis. 2013;33:547–658.
Article CAS Google Scholar
Froestl W, Muhs A, Pfeifer A. Cognitive enhancers (nootropics). Part 1: drugs interacting with receptors. J Alzheimers Dis. 2012;32:793–887.
Froestl W, Pfeifer A, Muhs A. Cognitive enhancers (nootropics). Part 3: drugs interacting with targets other than receptors or enzymes disease-modifying drugs. J Alzheimers Dis. 2013;34:1–114.
Lanni C, Lenzken SC, Pascale A, Del Vecchio I, Racchi M, Pistoia F, et al. Cognition enhancers between treating and doping the mind. Pharmacol Res. 2008;57:196–213.
Carton L, Cabé N, Ménard O, Deheul S, Caous AS, Devos D, et al. Pharmaceutical cognitive doping in students: A chimeric way to get-a-head? Therapies. 2018;73:331–9.
Abad-Santos F, Novalbos-Reina J, Gallego-Sandin S, Garcia AG. Treatment of mild cognitive impairment: value of citicoline. Rev Neurol. 2002;7:657–82.
Google Scholar
Corkery J, Schifano F, Oyefeso A, Ghodse A, Tonia T, Naidoo V, et al. Overview of literature and information on “khat-related” mortality: a call for recognition of the issue and further research. Ann Ist Super Sanita. 2011;47:445–64.
PubMed Google Scholar
Perić I, Costina V, Djordjević S, Gass P, Findeisen P, Inta D, et al. Tianeptine modulates synaptic vesicle dynamics and favors synaptic mitochondria processes in socially isolated rats. Sci Rep. 2021;111(1):1–15.
Holgado D, Zandonai T, Zabala M, Hopker J, Perakakis P, Luque-Casado A, et al. Tramadol effects on physical performance and sustained attention during a 20-min indoor cycling time-trial: a randomised controlled trial. J Sci Med Sport. 2018;21:654–60.
Orsolini L, St John-Smith P, McQueen D, Papanti D, Corkery J, Schifano F. Evolutionary considerations on the emerging subculture of the e-psychonauts and the novel psychoactive substances: a comeback to the shamanism? Curr Neuropharmacol. 2017;15(5):731–7. https://doi.org/10.2174/1570159x15666161111114838 .
Damiri B, Safarini OA, Nazzal Z, et al. Eating disorders and the use of cognitive enhancers and psychostimulants among university students: a cross-sectional study. Neuropsychiatr Dis Treat. 2021;17:1633.
McDermott H, Lane H. Alonso M (2020) Working smart: the use of ‘cognitive enhancers’ by UK university students. J Further Higher Educ. 2020;45(2):270–83. https://doi.org/10.1080/0309877X20201753179 .
Nelson M, Jensen C, Lenton S. Study drug use among university students in Western Australia: results of a web survey and their policy and practice implications. Drug Alcohol Rev. 2021;40:530–9.
Plumber N, Majeed M, Ziff S, Thomas SE, Bolla SR, Gorantla VR. Stimulant usage by medical students for cognitive enhancement: a systematic review. Cureus. 2021;13(5):e15163. https://doi.org/10.7759/CUREUS.15163 .
Marsh S. Universities must do more to tackle use of smart drugs, say experts. 2017. https://www.theguardian.com/education/2017/may/10/universities-do-more-tackle-smart-drugs-say-experts-uk-exams . Accessed 10 Sep 2021
Burgard D, Fuller R, Becker B, Ferrell R, Dinglasan-Panlilio M. Potential trends in Attention Deficit Hyperactivity Disorder (ADHD) drug use on a college campus: wastewater analysis of amphetamine and ritalinic acid. Sci Total Environ. 2013;450–451:242–9.
Maier L, Ferris J, Winstock A. Pharmacological cognitive enhancement among non-ADHD individuals-A cross-sectional study in 15 countries. Int J Drug Policy. 2018;58:104–12.
Benson K, Flory K, Humphreys S, Lee L. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18:50–76.
Ponnet K, Tholen R, De Bruyn S, Wouters E, Van Ouytsel J, Walrave M, et al. Students’ stimulant use for cognitive enhancement: a deliberate choice rather than an emotional response to a given situation. Drug Alcohol Depend. 2021;218:108410. https://doi.org/10.1016/J.DRUGALCDEP.2020.108410 .
Singh I, Bard I, Jackson J. Robust resilience and substantial interest: a survey of pharmacological cognitive enhancement among university students in the UK and Ireland. PLoS One. 2014;9:e105969.
Yamamoto M, Ishii Y. Questionnaire survey concerning pharmacological cognitive enhancement among undergraduates [in Japanese]. Yakugaku Zasshi. 2020;140:1397–403.
Alrakaf FA, Binyousef FH, Altammami AF, Alharbi AA, Shadid A, Alrahili N. Illicit stimulant use among medical students in Riyadh, Saudi Arabia. Cureus. 2020;12(1):e6688. https://doi.org/10.7759/CUREUS.6688 .
Micoulaud-Franchi J-A, MacGregor A, Fond G. A preliminary study on cognitive enhancer consumption behaviors and motives of French Medicine and Pharmacology students. Eur Rev Med Pharmacol Sci. 2014;18:1875–8.
de Oliveira Cata Preta B, Miranda V, Bertoldi A. Psychostimulant use for neuroenhancement (smart drugs) among college students in Brazil. Subst Use Misuse. 2020;55:613–21.
Zeeuws I, Soetens E. Verbal memory performance improved via an acute administration of D-amphetamine. Hum Psychopharmacol. 2007;22:279–87.
Dolder P, Strajhar P, Vizeli P, Odermatt A, Liechti M. Acute effects of lisdexamfetamine and D-amphetamine on social cognition and cognitive performance in a placebo-controlled study in healthy subjects. Psychopharmacology. 2018;235:1389–402.
Roberts C, Jones A, Sumnall H, Gage S, Montgomery C. How effective are pharmaceuticals for cognitive enhancement in healthy adults? A series of meta-analyses of cognitive performance during acute administration of modafinil, methylphenidate and D-amphetamine. Eur Neuropsychopharmacol. 2020;38:40–62.
Hoots J, Webber H, Nunez C, Cooper J, Lopez-Gamundi P, Lawlor V, et al. Acute drug effects differentially predict desire to take dextroamphetamine again for work and recreation. Psychopharmacology. 2021;238:2815–26.
Wardle MC, Hart AB, Palmer AA, De Wit H. Does COMT genotype influence the effects of d-amphetamine on executive functioning? Genes Brain Behav. 2013;12:13.
Schifano F. A bitter pill. Overview of ecstasy (MDMA, MDA) related fatalities. Psychopharmacology. 2004;173:242–8.
de Sousa Fernandes Perna E, Theunissen E, Kuypers K, Heckman P, de la Torre R, Farre M, et al. Memory and mood during MDMA intoxication, with and without memantine pretreatment. Neuropharmacology. 2014;87:198–205.
Linssen A, Sambeth A, Vuurman E, Riedel W. Cognitive effects of methylphenidate in healthy volunteers: a review of single dose studies. Int J Neuropsychopharmacol. 2014;17:961–77.
Batistela S, Bueno O, Vaz L, Galduróz J. Methylphenidate as a cognitive enhancer in healthy young people. Dement Neuropsychol. 2016;10:134–42.
Klinge C, Shuttleworth C, Muglia P, Nobre A, Harmer C, Murphy S. Methylphenidate enhances implicit learning in healthy adults. J Psychopharmacol. 2018;32:70–80.
Repantis D, Bovy L, Ohla K, Kühn S, Dresler M. Cognitive enhancement effects of stimulants: a randomized controlled trial testing methylphenidate, modafinil, and caffeine. Psychopharmacology. 2021;238:441–51.
Kumar A, Gupta V, Sharma S. Donepezil. Profiles Drug Subst Excipients Relat Methodol. 2021;35:117–50.
Yesavage J, Mumenthaler M, Taylor J, Friedman L, O’Hara R, Sheikh J, et al. Donepezil and flight simulator performance: effects on retention of complex skills. Neurology. 2002;59:123–5.
Beglinger L, Gaydos B, Kareken D, Tangphao-Daniels O, Siemers E, Mohs R. Neuropsychological test performance in healthy volunteers before and after donepezil administration. J Psychopharmacol. 2004;18:102–8.
Ginani G, Tufik S, Bueno O, Pradella-Hallinan M, Rusted J, Pompéia S. Acute effects of donepezil in healthy young adults underline the fractionation of executive functioning. J Psychopharmacol. 2011;25:1508–16.
Zaninotto A, Bueno O, Pradella-Hallinan M, Tufik S, Rusted J, Stough C, et al. Acute cognitive effects of donepezil in young, healthy volunteers. Hum Psychopharmacol. 2009;24:453–64.
Balsters JH, O’Connell RG, Martin MP, et al. Donepezil impairs memory in healthy older subjects: behavioural, EEG and simultaneous EEG/fMRI biomarkers. PLoS One. 2011;6(9):e24126. https://doi.org/10.1371/JOURNAL.PONE.0024126 .
Yasar S, Goldberg J, Goldberg S. Are metabolites of l-deprenyl (selegiline) useful or harmful? Indications from preclinical research. J Neural Transm. 1996;Suppl 48:61–73.
Tatton W, Wadia J, Ju W, Chalmers-Redman R, Tatton N. (-)-Deprenyl reduces neuronal apoptosis and facilitates neuronal outgrowth by altering protein synthesis without inhibiting monoamine oxidase. J Neural Transm. 1996;Suppl 48:45–59.
Gelowitz D, Richardson J, Wishart T, Yu P, Lai C. Chronic L-deprenyl or L-amphetamine: equal cognitive enhancement, unequal MAO inhibition. Pharmacol Biochem Behav. 1994;47:41–5.
Goverdhan P, Sravanthi A, Mamatha T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimers Dis. 2012;2012:974013. https://doi.org/10.1155/2012/974013 .
Zhu J, Hamm R, Reeves T, Povlishock J, Phillips L. Postinjury administration of L-deprenyl improves cognitive function and enhances neuroplasticity after traumatic brain injury. Exp Neurol. 2000;166:136–52.
Yang H, Han W, Li H. Efficacy and safety of MAO-B inhibitor versus donepezil in Chinese elderly stroke patients with Alzheimer disease: a potential therapeutic option. Pak J Pharm Sci. 2020;33:1349–54.
CAS PubMed Google Scholar
Dongsoo K. Practical use and risk of modafinil, a novel waking drug. Environ Health Toxicol. 2012;27:e2012007.
Hashemian SM, Farhadi T. A review on modafinil: the characteristics, function, and use in critical care. J Drug Assess. 2020;9:82.
Mereu M, Bonci A, Newman A, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology. 2013;229:415–34.
Kredlow M, Keshishian A, Oppenheimer S, Otto M. The efficacy of modafinil as a cognitive enhancer: a systematic review and meta-analysis. J Clin Psychopharmacol. 2019;39:455–61.
Fernández A, Mascayano F, Lips W, Painel A, Norambuena J, Madrid E. Effects of modafinil on attention performance, short-term memory and executive function in university students: a randomized trial. Medwave. 2015;15:e6166.
Turner D, Robbins T, Clark L, Aron A, Dowson J, Sahakiandc B. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165:260–9.
Gilleen J, Michalopoulou P, Reichenberg A, Drake R, Wykes T, Lewis S, et al. Modafinil combined with cognitive training is associated with improved learning in healthy volunteers—a randomised controlled trial. Eur Neuropsychopharmacol. 2014;24:529–39.
Battleday RM, Brem AK. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review. Eur Neuropsychopharmacol. 2015;25:1865–81.
Müller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ. Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology. 2013;64:490.
Lees J, Michalopoulou P, Lewis S, et al. Modafinil and cognitive enhancement in schizophrenia and healthy volunteers: the effects of test battery in a randomised controlled trial. Psychol Med. 2017;47:2358–68.
Corazza O, Bersani FS, Brunoro R, Valeriani G, Martinotti G, Schifano F. The diffusion of performance and image-enhancing drugs (PIEDs) on the internet: the abuse of the cognitive enhancer piracetam. Subst Use Misuse. 2014;49(14):1849–56. https://doi.org/10.3109/10826084.2014.912232 .
Winblad B. Piracetam: a review of pharmacological properties and clinical uses. CNS Drug Rev. 2005;11:169–82.
Brandão F, Cadete-Leite A, Andrade J, Madeira M, Paula-Barbosa M. Piracetam promotes mossy fiber synaptic reorganization in rats withdrawn from alcohol. Alcohol. 1996;13:239–49.
Ahmed AH, Oswald RE. Piracetam defines a new binding site for allosteric modulators of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J Med Chem. 2010;53:2197.
Catalani V, Botha M, Corkery JM, Guirguis A, Vento A, Schifano F. Cognitive enhancers: computational models on benzodiazepines and racetams identified online. [webinar presentation]. Analytical Toxicology for Novel Psychoactive Substances Webinar, ISSED 2021, online. https://issed.net/ .
Michel C, Lehmann D. Single doses of piracetam affect 42-channel event-related potential microstate maps in a cognitive paradigm. Neuropsychobiology. 1993;28:212–21.
Kondakor I, Michel C, Wackermann J, Koenig T, Tanaka H, Peuvot J, et al. Single-dose piracetam effects on global complexity measures of human spontaneous multichannel EEG. Int J Psychophysiol. 1999;34:81–7.
Itil TM, Menon GN, Songar A, Itil KZ. CNS pharmacology and clinical therapeutic effects of oxiracetam—PubMed. Clin Neuropharmacol. 1986;3:70–2.
Buettelmann B, Ballard T, Gasser R, Fischer H, Hernandez M, Knoflach F, et al. Imidazo[1,5-a][1,2,4]-triazolo[1,5-d][1,4]benzodiazepines as potent and highly selective GABAA alpha5 inverse agonists with potential for the treatment of cognitive dysfunction. Bioorg Med Chem Lett. 2009;19:5958–61.
Gualtieri F. Unifi nootropics from the lab to the web: a story of academic (and industrial) shortcomings. J Enzyme Inhib Med Chem. 2016;31:187–94.
Martini E, Ghelardini C, Dei S, Guandalini L, Manetti D, Melchiorre M, et al. Design, synthesis and preliminary pharmacological evaluation of new piperidine and piperazine derivatives as cognition-enhancers. Bioorg Med Chem. 2008;16:1431–43.
Martino MV, Guandalini L, Di Cesare ML, Menicatti M, Bartolucci G, Dei S, et al. Piperazines as nootropic agents: new derivatives of the potent cognition-enhancer DM235 carrying hydrophilic substituents. Bioorg Med Chem. 2017;25:1795–803.
Galeotti N, Ghelardini C, Pittaluga A, Pugliese A, Bartolini A, Manetti D, et al. AMPA-receptor activation is involved in the antiamnesic effect of DM 232 (unifiram) and DM 235 (sunifiram). Naunyn Schmiedebergs Arch Pharmacol. 2003;368:538–45.
Peltier MR, Sofuoglu M. Pharmacological cognitive enhancers. In: Verdejo-Garcia A, editor. Cognition and addiction. New York: Elsevier; 2020. p. 303–20.
Chapter Google Scholar
Ricci G. Pharmacological human enhancement: an overview of the looming bioethical and regulatory challenges. Front Psychiatry. 2020;11:53. https://doi.org/10.3389/FPSYT.2020.00053 .
Fond G, Micoulaud-Franchi J, Macgregor A, Richieri R, Lancon C, Repantis D. Neuroenhancement in healthy adults, Part I: pharmaceutical cognitive enhancement: a systematic review. J Clin Res Bioeth. 2015;6:1–15. https://doi.org/10.4172/2155-9627.1000213 .
Schifano F, Orsolini L, Duccio Papanti G, Corkery JM. Novel psychoactive substances of interest for psychiatry. World Psychiatry. 2015;14:15–26.
Martinotti G, Merino Del Villar C, Cordoba GL, Tubau A, Sánchez C, Di Carlo F, et al. Club drugs and psychiatric sequelae: an issue of vulnerability and previous psychiatric history. Int J Environ Res Public Health. 2021;18(13):6944. https://doi.org/10.3390/IJERPH18136944 .
Schifano F, Orsolini L, Papanti D, Corkery J. NPS: medical consequences associated with their intake. Curr Top Behav Neurosci. 2017;32:351–80.
Márquez J, Campos-Sandoval JA, Peñalver A, Matés JM, Segura JA, Blanco E, et al. Glutamate and brain glutaminases in drug addiction. Neurochem Res. 2017;42(3):846–57.
Arnsten AFT, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–84.
Urban K, Li Y, Gao W. Treatment with a clinically-relevant dose of methylphenidate alters NMDA receptor composition and synaptic plasticity in the juvenile rat prefrontal cortex. Neurobiol Learn Mem. 2013;101:65–74.
Urban KR, Gao W-J. Performance enhancement at the cost of potential brain plasticity: neural ramifications of nootropic drugs in the healthy developing brain. Front Syst Neurosci. 2014;8:38.
Whitehurst LN, Mednick SC. Psychostimulants may block long-term memory formation via degraded sleep in healthy adults. Neurobiol Learn Mem. 2021;178:107342.
Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients—results of the COMPAS study. Pharmacopsychiatry. 2020;53:263–71.
Weiss MD, Cutler AJ, Kollins SH, Donnelly GAE. Efficacy and safety of a long-acting multilayer-release methylphenidate formulation (PRC-063) in the treatment of adolescent attention-deficit/hyperactivity disorder: a randomized, double-blind clinical trial with a 6-month open-label extension. J Child Adolesc Psychopharmacol. 2021;31(9):610–22. https://doi.org/10.1089/CAP.2021.0034 .
Koren G, Korn L. The use of methylphenidate for cognitive enhancement in young healthy adults: the clinical and ethical debates. J Clin Psychopharmacol. 2021;41:100–2.
Carlier J, Giorgetti R, Varì M, Pirani F, Ricci G, Busardò F. Use of cognitive enhancers: methylphenidate and analogs. Eur Rev Med Pharmacol Sci. 2019;23:3–15.
Tomen D. Do Brain Enhancing “Drugs” Work? Know the Risks. 2020. https://nootropicsexpert.com/do-brain-enhancing-drugs-work-know-the-risks/ . Accessed 30 Sep 2021
Schneider LS, Tariot PN, Goldstein B. Therapy with l-deprenyl (selegiline) and relation to abuse liability. Clin Pharmacol Ther. 1994;56:750–6.
Greenblatt K, Adams N. Modafinil. StatPearls; 2020. https://www.ncbi.nlm.nih.gov/books/NBK531476/ . Accessed 3 Mar 2022.
Kaplan S, Goehring EL, Melamed-Gal S, Nguyen-Khoa BA, Knebel H, Jones JK. Modafinil and the risk of cardiovascular events: findings from three US claims databases. Pharmacoepidemiol Drug Saf. 2018;27:1182–90.
Van Puyvelde M, Van Cutsem J, Lacroix E, Pattyn N. A state-of-the-art review on the use of modafinil as a performance-enhancing drug in the context of military operationality. Mil Med. 2022;187(1–2):52–64. https://doi.org/10.1093/MILMED/USAB398 .
Murillo-Rodríguez E, Barciela Veras A, Barbosa Rocha N, Budde H, Machado S. An overview of the clinical uses, pharmacology, and safety of modafinil. ACS Chem Neurosci. 2018;9:151–8.
Hall M, Forshaw M, Montgomery C. Being limitless: a discursive analysis of online accounts of modafinil use. In: Hall M, Forshaw M, Montgomery C, editors. Chemically Modified minds: substance use for cognitive enhancement. Berlin: SpringerLink; 2021. p. 81–99.
Savarese M, Di Perri MC. Excessive sleepiness in shift work disorder: a narrative review of the last 5 years. Sleep Breath. 2019;24:297–310.
Benjamin M. The ultimate guide to armodafinil in 2021. UK nootropics review. 2020. https://www.brainzyme.com/blogs/nootropics-uk/armodafinil . Accessed 16 Nov 2021.
Daubner J, Arshaad MI, Henseler C, Hescheler J, Ehninger D, Broich K, et al. Pharmacological neuroenhancement: current aspects of categorization, epidemiology, pharmacology, drug development, ethics, and future perspectives. Neural Plast. 2021;2021:8823383. https://doi.org/10.1155/2021/8823383 .
Li D-D, Zhang Y-H, Zhang W, Zhao P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of alzheimer’s disease. Front Neurosci. 2019;13:472. https://doi.org/10.3389/FNINS.2019.00472 .
Sharma A, Couture J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann Pharmacother. 2014;48:209–25.
Kallweit U, Bassetti CL. Pharmacological management of narcolepsy with and without cataplexy. Expert Opin Pharmacother. 2017;18:809–17.
Fond G, Micoulaud-Franchi JA, Brunel L, Macgregor A, Miot S, Lopez R, et al. Innovative mechanisms of action for pharmaceutical cognitive enhancement: a systematic review. Psychiatry Res. 2015;229:12–20.
de Jongh R, Bolt I, Schermer M, Olivier B. Botox for the brain: enhancement of cognition, mood and pro-social behavior and blunting of unwanted memories. Neurosci Biobehav Rev. 2008;32:760–76.
Maier L, Wunderli M, Vonmoos M, Römmelt A, Baumgartner M, Seifritz E, et al. Pharmacological cognitive enhancement in healthy individuals: a compensation for cognitive deficits or a question of personality? PLoS One. 2015;10(6):e0129805. https://doi.org/10.1371/JOURNAL.PONE.0129805 .
Zaami S, Minutillo A, Sirignano A, Marinelli E. Effects of appearance- and performance-enhancing drugs on personality traits. Front Psychiatry. 2021;12:730167.
Mohamed A, Sahakian B. The ethics of elective psychopharmacology. Int J Neuropsychopharmacol. 2012;15:559–71.
Sahakian BJ, Bruhl AB, Cook J, et al. The impact of neuroscience on society: cognitive enhancement in neuropsychiatric disorders and in healthy people. Philos Trans R Soc B Biol Sci. 2015;370(1677):214. https://doi.org/10.1098/RSTB.2014.0214 .
Beyer C, Staunton C, Moodley K. The implications of methylphenidate use by healthy medical students and doctors in South Africa. BMC Med Ethics. 2014;15:20. https://doi.org/10.1186/1472-6939-15-20 .
Jaha R, Kolak T, Helać H, Ćesir H, Sarajlić E, Spahić M. Smartdrugs: Mechanisms of Action and Ethical Issues. In: Badnjevic A, GurbetaPokvić L (eds). CMBEBIH 2021. IFMBE Proceedings, Vol 84. Cham: Springer; 2021. pp 462–468. https://doi.org/10.1007/978-3-030-73909-6_53 .
Ram S, Russell B, Kirkpatrick C, Stewart K, Scahill S, Henning M, et al. Professionals’ attitudes towards the use of cognitive enhancers in academic settings. PLoS One. 2020;15:e0241968.
Lakhan SE, Kirchgessner A. Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav. 2012;2:661–77.
Alcohol and Drug Foundation. Nootropics. 2019. https://adf.org.au/drug-facts/cognitive-enhancers/ . Accessed 29 Sep 2021.
Kapur A. Is methylphenidate beneficial and safe in pharmacological cognitive enhancement? CNS Drugs. 2020;34:1045–62.
Colzato LS, Hommel B, Beste C. The downsides of cognitive enhancement. Neuroscientist. 2021;27(4):322–30. https://doi.org/10.1177/1073858420945971 .
Rudra P. Ethical underpinning and implications of “nootropic” concept. Acta Univ Lodz Folia Philos Ethica Aestheticaract. 2018;32:31–45.
Mann SP, Sahakian BJ. Modafinil and the increasing lifestyle use of smart drugs by healthy people: neuroethical and societal issues—PsycNET. In: Johnson LSM, Rommelfanger KS, editors. Routledge handbook of neuroethics. Routledge: Taylor & Francis Group; 2018. p. 134–49.
De Castro B, Brandão E. Circulation of information on drugs and other substances to increase cognitive performance: a study of a Brazilian blog (2015–2017) [in Spanish]. Salude Collect. 2020;16:e2514. https://doi.org/10.18294/sc.2020.2514 .
Orsolini L, Francesconi G, Papanti D, Giorgetti A, Schifano F. Profiling online recreational/prescription drugs’ customers and overview of drug vending virtual marketplaces. Hum Psychopharmacol. 2015;30:302–18.
Keppel Hesselink JM. Smart drugs’ enticements on the Internet. Ned Tijdschr Geneeskd. 1998;142:977–80.
Bojanić I, Sund ER, Bjerkeset O, Sivertsen B, Sletvold H. Psychological distress and use of psychotropic drugs among university students—the SHoT study, Norway. Front Psychiatry. 2021;12:717955.
Racine E, Sattler S, Boehlen W. Cognitive enhancement: unanswered questions about human psychology and social behavior. Sci Eng Ethics. 2021;272(27):19.
Download references
Author information
Authors and affiliations.
Psychopharmacology, Drug Misuse and Novel Psychoactive Substances Research Unit, School of Life and Medical Sciences, University of Hertfordshire, College Lane Campus, Hatfield, UK
Fabrizio Schifano, Valeria Catalani, Safia Sharif, John Martin Corkery, Davide Arillotta, Suzanne Fergus & Amira Guirguis
East London Foundation Trust (ELFT), Newham Early Intervention Service, London, UK
Flavia Napoletano
Department of Mental Health, ASL Roma 2, Rome, Italy
Alessandro Vento
Addictions’ Observatory (ODDPSS), Rome, Italy
Department of Psychology, Guglielmo Marconi University, Rome, Italy
Swansea University Medical School, Institute of Life Sciences 2, Swansea University, Singleton Park, Swansea, UK
Amira Guirguis
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Fabrizio Schifano .
Ethics declarations
No financial assistance was used to assist in the preparation of the manuscript.
Conflicts of interest
Fabrizio Schifano, Valeria Catalani, Safia Sharif, Flavia Napoletano, John Martin Corkery, Davide Arillotta, Suzanne Fergus, Alessandro Vento, and Amira Guirguis declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Consent for publication, availability of data and material, code availability, author contributions.
FS conceived the idea for the manuscript and coordinated the project. VC, SS and FN carried out data collection and systematization. VC and FS analyzed the data. FS drafted the manuscript, and JMC, DA, SF, AV and AG critically reviewed the manuscript.
Additional information
The original article has been updated: Due to third author update.
Rights and permissions
Reprints and permissions
About this article
Schifano, F., Catalani, V., Sharif, S. et al. Benefits and Harms of ‘Smart Drugs’ (Nootropics) in Healthy Individuals. Drugs 82 , 633–647 (2022). https://doi.org/10.1007/s40265-022-01701-7
Download citation
Accepted : 07 March 2022
Published : 02 April 2022
Issue Date : April 2022
DOI : https://doi.org/10.1007/s40265-022-01701-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
Chemical Communications
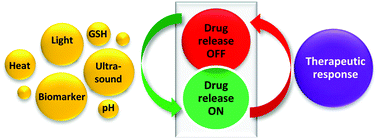
Smart drug delivery systems: from fundamentals to the clinic.
* Corresponding authors
a Departamento de Farmacia y Tecnología Farmacéutica, Universidad de Santiago de Compostela, 15782-Santiago de Compostela, Spain E-mail: [email protected]
Forty years after the first reports on stimuli-responsive phase transitions in synthetic hydrogels, the first medicines based on responsive components are approaching the market. Sensitiveness to internal or external signals of the body can be achieved by means of materials (mostly polymers, but also lipids and metals) that modify their properties as a function of the intensity of the signal and that enable the transduction into changes in the delivery system that affect its ability to host/release a therapeutic substance. Integration of responsive materials into implantable depots, targetable nanocarriers and even insertable medical devices can endow them with activation-modulated and feedback-regulated control of drug release. This review offers a critical overview of therapeutically-interesting stimuli to trigger drug release and the evolution of responsive materials suitable as functional excipients, illustrated with recent examples of formulations in clinical trials or already commercially available, which can provide a perspective on the current state of the art on smart drug delivery systems.
Article information
Download citation, author version available, permissions.
C. Alvarez-Lorenzo and A. Concheiro, Chem. Commun. , 2014, 50 , 7743 DOI: 10.1039/C4CC01429D
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author, advertisements.
Nootropics as Cognitive Enhancers: Types, Dosage and Side Effects of Smart Drugs
Affiliation.
- 1 Department of Agroenvironmental Chemistry and Plant Nutrition, Czech University of Life Sciences Prague, Kamýcká 129, 165 00 Prague, Czech Republic.
- PMID: 36014874
- PMCID: PMC9415189
- DOI: 10.3390/nu14163367
Nootropics, also known as "smart drugs" are a diverse group of medicinal substances whose action improves human thinking, learning, and memory, especially in cases where these functions are impaired. This review provides an up-to-date overview of the potential effectiveness and importance of nootropics. Based on their nature and their effects, this heterogeneous group of drugs has been divided into four subgroups: classical nootropic compounds, substances increasing brain metabolism, cholinergic, and plants and their extracts with nootropic effects. Each subgroup of nootropics contains several main representatives, and for each one, its uses, indications, experimental treatments, dosage, and possible side effects and contraindications are discussed. For the nootropic plant extracts, there is also a brief description of each plant representative, its occurrence, history, and chemical composition of the medicinal part. Lastly, specific recommendations regarding the use of nootropics by both ill and healthy individuals are summarized.
Keywords: Panax ginseng; Paullinia cupana; antioxidant activity; ayurvedic; brain injury; learning; memory; nootropics; piracetam; smart drugs.
Publication types
- Drug-Related Side Effects and Adverse Reactions*
- Nootropic Agents* / therapeutic use
- Nootropic Agents
Grants and funding
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 02 March 2016
Smart drugs: A dose of intelligence
- Amber Dance 1
Nature volume 531 , pages S2–S3 ( 2016 ) Cite this article
52k Accesses
22 Citations
133 Altmetric
Metrics details
- Medical research
As mind sports becomes the new frontier for doping concerns, research is exploring whether users really get any value from 'smart drugs'.
In August 2015, 80 professional video gamers from around the world gathered for the ESL One Cologne competition in Germany. With US$250,000 in prize money up for grabs, pressure was high, and competition organizer ESL wanted to ensure fair play. At some point during the two-day event, a random selection of players received a tap on the shoulder and were escorted to a discreet back room where a physician awaited.

For the first time in its 16-year history, ESL was taking saliva samples on its lookout for dope. Smart drugs were allegedly circulating, helping players to get in the zone. “Just like in normal sports, it's not OK to win because you took a pill,” says Anna Rozwandowicz, ESL's director of communications and the first head of its anti-doping initiative. That weekend, all the tests came back clean.
E-athletes aren't the only ones allegedly popping pills to try to enhance their mental faculties. Use of the drugs seems to be common, although finding firm data is not easy. In 2014, a survey of British and Irish students reported that more than 3% currently used prescription medications as cognitive enhancers 1 . A 2013 survey of surgeons found that nearly 20% had used medication for cognitive enhancement at least once 2 . And an informal survey ( go.nature.com/xmlrn2 ) from 2008 reported that a similar proportion of Nature readers had used medications off-label to improve memory or concentration.
Many smart drugs are prescription medications either purchased illegally or used off-label. Top choices include Adderall (amphetamine) and Ritalin (methylphenidate) — treatments for attention-deficit hyperactivity disorder (ADHD) — and modafinil, which is a medication for sleep disorders such as narcolepsy. In people with ADHD or sleep disorders, these drugs can raise brain function so that it matches that of healthy people. But it is not clear whether the same medications can push a neurologically healthy, well-rested individual onto a higher cognitive plane. There is also the question of side effects. Despite these uncertainties, the apparently widespread use of neuroenhancers has prompted an ethical debate about whether their use is fair in school exams or mental games.
Like a boss
They don't really live up to the name smart pills
It is hard to say just how much these medications help an average person. Amphetamines improve focus and can make dull tasks seem interesting. So they might change a student's perspective from, 'Ugh, chemistry', to 'Ooh! Carbon bonds!' — even though that student is not any brighter. “They don't really live up to the name smart pills,” says Martha Farah, a cognitive neuroscientist at the University of Pennsylvania in Philadelphia. “Nothing that would turn you from a B to an A student or suddenly give you winning business ideas.”
It's still not clear precisely how these drugs produce their effects. Adderall and Ritalin are the best understood. Their main effects seem to relate to the neurotransmitters noradrenaline and dopamine, each of which mediates several effects, including attention and reward. Normally, a neuron releases these neurotransmitters as a message, telling other neurons to fire or stay quiet. Once the signal has been received, the first neuron re-absorbs the neurotransmitters. These medications block that re-uptake, so that the signals persist. Amphetamines also have other actions, such as preventing the breakdown of neurotransmitters.
Understanding of the cognitive-enhancement mechanism of modafinil is more sketchy. The drug affects “pretty much every major neurotransmitter in the brain”, says Ruairidh Battleday, a neuroscientist at the University of California, Berkeley. These include dopamine and noradrenaline, so part of its effect is probably similar to that of Adderall and Ritalin.
Extra neurotransmitters help parts of the brain to communicate better, particularly the prefrontal cortex, which neuroscientist Kimberly Urban calls the brain's “boss”. When noradrenaline and dopamine are present in the right amounts, the boss is an effective manager, explains Urban, who works at the Children's Hospital of Philadelphia. Too few neurotransmitters, and the boss is sluggish; too many, and it gets overwhelmed. The goal of treatments for ADHD and narcolepsy is to get the boss to the peak of function. Healthy users hope that they can raise their boss's peak.
People seeking a chemical boost do have legal options. “By far the most commonly used neurocognitive enhancers are nicotine and caffeine,” says Peter Morgan, a psychiatrist at Yale University School of Medicine in New Haven, Connecticut. Instead of blocking reuptake, caffeine stimulates the release of extra noradrenaline and dopamine; its effects aren't as strong or as long-lasting as those provided by the other drugs. Nicotine mimics the neurotransmitter acetylcholine, which affects learning and memory. And no one bans people from pumping up their brains by smoking or drinking coffee during competitions or before an exam.
Unclear benefits
Researchers are attempting to quantify the effects of prescription neuroenhancers in healthy people. In one study, Stefano Sensi, a neurologist at G. d'Annunzio University of Chieti–Pescara in Italy, and his team asked 26 university students to take an intelligence test. They then gave each volunteer either a dose of modafinil or a placebo before re-administering the test 3 . For the test, called Raven's matrices, participants were required to complete the ninth pattern in a 3 × 3 geometric puzzle. They were scored on the number of grids that they answered correctly. Solving the puzzle requires quick and flexible thinking — called fluid intelligence.

Results were mixed and depended on the difficulty of the matrices. Modafinil made no difference on the easiest or the hardest puzzles. The drug did increase scores for the grids of medium difficulty, mostly for those who scored low in the pre-drug test; it made little difference to participants who nailed the matrices on their first try.
Sensi's work was among 24 papers included in a 2015 review of modafinil in healthy people 4 . The studies used a variety of cognitive tests, and the review found that, on average, modafinil did seem to help — particularly with decision-making, planning and fluid intelligence. The more complex the task, the more that modafinil helped. “On the basis of the evidence,” says Battleday, “modafinil is improving people's performance.” But the results were not uniformly positive. Not every test showed benefits and, in a couple, the drug seems to have stunted creativity.
The review authors also noted that many cognitive tests had been designed to assess impairment, not enhancement. For example, people with a brain injury or dementia may struggle with a clock-drawing test, but someone with normal cognition will usually get it right — leaving no room for smart drugs to assist. Psychologists have few options to adequately measure cognition in healthy people, says review co-author Anna-Katharine Brem, a neuropsychologist at the University of Oxford, UK.
As with any mind-altering drug (caffeine and nicotine included) addiction or dependence are concerns. People who take drugs for ADHD do not seem to get hooked, says James McGough, a child and adolescent psychiatrist at the University of California, Los Angeles. However, he does not know if the same drugs might prove addictive in healthy people. After all, Adderall is an amphetamine, which has established addictive properties. Ritalin and modafinil seem to be less addictive, says Urban, but that does not mean that regular use is without risk. Morgan points out that regular use of coffee and cigarettes causes consumers' brains to adapt so that they need the stimulant just to function at their normal cognitive level. He suspects, the same might occur with smart drugs, even if users lack the compulsive craving that characterizes addiction.
As for long-term effects, nobody knows. Urban and colleagues' experiments in rats indicate that Ritalin could be bad for developing brains 5 . The researchers treated both adults and juveniles with one milligram per kilogram body weight, which is within the normal range for human treatment. In the grown-up rats, the drug increased nerve firing in their prefrontal cortex. But in the 15-day-old rats, equivalent to a preteen human, firing went down. If Urban stopped the drug, the effects went away. But when she tripled the dosage — equivalent to a high, but not unheard of, human prescription — the firing rates stayed low even 70 days after the treatment stopped.
The neurotransmitters that the medications are known to target, noradrenaline and dopamine, are crucial regulators of brain maturation during puberty, Urban explains. Although the drugs don't seem to cause problems in teenagers with ADHD, they might throw off development of a healthy brain. She speculates that the poor firing patterns observed in the rats might translate to problems with working memory and flexible thinking in people. For example, someone might have a hard time finding a new route to work if their usual path is blocked. Indeed, she says, healthy children who take too much Ritalin can exhibit “extreme perseverance”— for example, being unable to pause a video game when it's time for dinner, persisting with one topic of conversation without being able to switch gears, or feeling emotions such as anger for a longer time than a situation warrants.
Smart moral compass
Smart drugs are still primitive, Sensi says. They temporarily alter multiple neurotransmitters, so they aren't very specific. A better approach, he suggests, could be to develop drugs that would promote nerve-cell growth or the rewiring of the brain, inducing changes that would permanently enhance thinking.
However, the current medications are still potent enough to raise ethical questions. One such concern revolves around social equality. Not everyone has equal access to smart drugs, and there is a danger that only the privileged will be able to get ahead with amped-up cognitive powers. The result would be yet another force widening the gap between the haves and the have-nots, says Nita Farahany, a bioethicist at Duke University in Durham, North Carolina. Or, there might be a sort of arms race, with people taking ever more advanced smart drugs just to keep up. At her own institution, Farahany says, students were so worried about brain-boosters that the university amended its honour code in 2011 to state that “the unauthorized use of prescription medication to enhance academic performance” was a form of cheating.
For now, at least, even the scientists who study smart drugs aren't relying on them. Most of those interviewed for this article said that they stick to coffee, tea or energy drinks. Morgan, for his part, suggested that the same cognitive benefits can be achieved by simply taking a refreshing nap.
Singh, I., Bard, I. & Jackson, J. PLoS ONE 9 , e105969 (2014).
Article ADS Google Scholar
Franke, A. G. et al. BMC Med. 11 , 102 (2013).
Article Google Scholar
Esposito, R. et al. PLoS ONE 8 , e69224 (2013).
Article ADS CAS Google Scholar
Battleday, R. M. & Brem, A.-K. Eur. Neuropsychopharmcol. 25 , 1865–1881 (2015).
Article CAS Google Scholar
Urban, K. R., Waterhouse, B. D. & Gao, W.-J. Biol. Psychiatry 72 , 880–888 (2012).
Download references

Author information
Authors and affiliations.
Amber Dance is a freelance science writer based in Los Angeles, California.,
Amber Dance
You can also search for this author in PubMed Google Scholar
Related links
Related links in nature research.
Towards responsible use of cognitive-enhancing drugs by the healthy
Neuroscience: Drugs to build a better brain
Turbocharging the Brain
Do “Smart Pills” Really Make You Smart?
Related external links
Raven's Matrices intelligence test
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Dance, A. Smart drugs: A dose of intelligence. Nature 531 , S2–S3 (2016). https://doi.org/10.1038/531S2a
Download citation
Published : 02 March 2016
Issue Date : 03 March 2016
DOI : https://doi.org/10.1038/531S2a
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A systematic review of the co-occurrence of gaming disorder and other potentially addictive behaviors.
- Tyrone L. Burleigh
- Mark D. Griffiths
- Daria J. Kuss
Current Addiction Reports (2019)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Towards Smart Drug Delivery: Exploration of a Molecularly Imprinted Polymer Network
- Master of Science
- Molecular Science
Granting Institution
Lac thesis type, usage metrics.

- Drug discovery, design and delivery

Embargoed and Restricted Access
Reason: Under embargo until October 2020. After this date a copy can be supplied under Section 51(2) of the Australian Copyright Act 1968 by submitting a document delivery request through your library
Smart Drug Delivery And Image-Guided Therapy for Cancer with Modified Carbonate Apatite Nanoparticles
Campus location, principal supervisor, additional supervisor 1, additional supervisor 2, additional supervisor 3, year of award, department, school or centre, degree type, usage metrics.

- Biomedical engineering not elsewhere classified
- Nanomedicine
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Evid Based Complement Alternat Med
- v.2016; 2016

Establishing Natural Nootropics: Recent Molecular Enhancement Influenced by Natural Nootropic
Noor azuin suliman.
1 Department of Human Anatomy, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 Serdang, Malaysia
Che Norma Mat Taib
Mohamad aris mohd moklas, mohd ilham adenan.
2 Atta-ur-Rahman Institute for Natural Product Discovery, Aras 9 Bangunan FF3, UiTM Puncak Alam, Bandar Baru Puncak Alam, 42300 Selangor Darul Ehsan, Malaysia
Mohamad Taufik Hidayat Baharuldin
Rusliza basir.
Nootropics or smart drugs are well-known compounds or supplements that enhance the cognitive performance. They work by increasing the mental function such as memory, creativity, motivation, and attention. Recent researches were focused on establishing a new potential nootropic derived from synthetic and natural products. The influence of nootropic in the brain has been studied widely. The nootropic affects the brain performances through number of mechanisms or pathways, for example, dopaminergic pathway. Previous researches have reported the influence of nootropics on treating memory disorders, such as Alzheimer's, Parkinson's, and Huntington's diseases. Those disorders are observed to impair the same pathways of the nootropics. Thus, recent established nootropics are designed sensitively and effectively towards the pathways. Natural nootropics such as Ginkgo biloba have been widely studied to support the beneficial effects of the compounds. Present review is concentrated on the main pathways, namely, dopaminergic and cholinergic system, and the involvement of amyloid precursor protein and secondary messenger in improving the cognitive performance.
1. Introduction
The “nootropic” or simplified as a “smart drug,” “brain booster,” or “memory enhancing drug,” is a common term that will tag along with the compound responsible for the enhancement of mental performance. By definition, nootropic is a compound that increases mental functions including memory, motivation, concentration, and attention [ 1 ]. There are two different nootropics: synthetic, a lab created compound such as Piracetam, and notable natural and herbal nootropics, such as Ginkgo biloba and Panax quinquefolius (American Ginseng).
Natural nootropics are proven in boosting the brain function while at the same time making the brain healthier. Nootropics act as a vasodilator against the small arteries and veins in the brain [ 2 ]. Introduction of natural nootropics in the system will increase the blood circulation to the brain and at the same time provide the important nutrient and increase energy and oxygen flow to the brain [ 3 ]. Despite the 3% weight of total body weight, the brain receives around 15% of the body's total blood supply and oxygen. In fact, the brain can only generate energy from burning the glucose [ 4 ], proving that neuron depends on the continuous supply of oxygen and nutrients.
In contrast to most of other cells in the body, neuron cannot be reproduced and is irreplaceable. The neuron cells are persistently expending the converted energy to maintain the repair of the cell compartments. The energy generated from the glucose is crucial for maintenance, electrical, and neurotransmitter purposes [ 5 ]. The effect of natural nootropics is also shown to reduce the inflammation occurrence in the brain [ 6 ]. The administration of nootropics will protect the brain from toxins and minimising the effects of brain aging. Effects of natural nootropics in improving the brain function are also contributed through the stimulation of the new neuron cell. As incentive from the new neuronal cell, the activity of the brain is increased, enhancing the thinking and memory abilities, thus increasing neuroplasticity [ 7 ].
Commercialised natural nootropics in the market are reacting at different mechanisms, thus affecting different parameters. Natural nootropics alter the concentration of existing neurotransmitters. Natural nootropics have been disclosed to stimulate the release of dopamine, uptake of choline, cholinergic transmission, function of α -amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor, turnover of phosphatidylinositol, and activity of phosphatase A2 [ 8 ]. Some of the natural nootropics act as a positive allosteric modulator for acetylcholine or glutamate receptor [ 9 ]. The release of neurotransmitter [ 10 ] and the increase activity of neurotransmitter [ 11 ] induced by natural nootropics facilitate the long-term potential (LTP) and improve synaptic transmission.
2. Suggested Molecular and Cellular Mechanisms
Establishment of new natural nootropic must consider the cellular and molecular mechanism of cognitive processes. A neuron has a known structural and functional plasticity or termed as synaptic plasticity, responsible for synaptic remodelling or known as cellular learning. Modulation in the molecular level in the neuron will alter the cognitive properties [ 12 ]. Through this review, there are few suggested mechanisms mediating the effects of nootropics in cognitive performance.
2.1. Glutaminergic Signalling
Glutamatergic transmission is an example of synaptic plasticity associated with LTP. Glutamate is an essential neurotransmitter involved in cognitive processes [ 13 ]. There are two different types of glutamate receptors: ionotropic (AMPA, kainite and NMDA receptors) and metabotropic receptors, distributed on the pre- and postsynaptic sites of the neuron. These receptors are responsible for neuronal network that allows the cognitive performance [ 14 ]. The release of glutamate will activate N-methyl-D-aspartate (NMDA) and AMPA receptors. AMPA receptor is responsible for synaptic transmission, while NMDA receptor is responsible for classic learning and memory [ 15 ]. The brain will respond by opening the Na + /K + ion channel and depolarising the cell membrane [ 16 ]. However, hyperactivity of glutamate receptors can cause oxidative stress to occur responding to the cognitive dysfunction [ 17 ].
Glutamate, which acts by activating NMDA receptor, is a main excitatory neurotransmitter related in cognition function [ 15 ]. The NMDA receptor is an ionotropic channel and distributes abundantly in the hippocampus, cortex, and thalamus [ 18 ] that assists the movement of Ca 2+ , Na + , and K + ions [ 19 ]. Activation of the NMDA receptor is reported to initiate the LTP observed in the hippocampus. LTP is part of the synaptic plasticity responsible for the physiological changes of cognitive functions [ 20 ]. LTP is remarkably studied in the hippocampus associated with learning and memory [ 21 ]. The increased Ca 2+ permeability and blockage of voltage-dependent Mg 2+ contribute to the synaptic plasticity and the formation of memory [ 19 ]. Increased Ca 2+ also affects the gene and protein expression for LTP and, subsequently, may lead to neurotoxicity due to overexcitation of glutamate observed in Alzheimer's disease [ 16 ]. Blockage of NMDA receptor displays the cognitive impairment in animal models. The models are mimicking the association between the receptor to dementia [ 22 ] and schizophrenia [ 23 ] diseases. Downregulated glutamate is observed in Alzheimer's disease [ 24 ] accompanied by the reduction of NMDA receptor in the hippocampus [ 25 ].
AMPA receptor, another type of ionotropic channel, is known to mediate the fast and immediate postsynaptic response to glutamate release and thus may contribute to synaptic plasticity [ 26 ]. The receptor can be found throughout the brain, especially in the thalamus, hypothalamus, cerebral cortex, hippocampus, basal ganglia, and cerebellum [ 18 ], being also permeable for Na + and K + [ 27 ]. Increased density of AMPA receptor in the hippocampus was shown to enhance the memory consolidation [ 28 ]. The use of AMPA modulator causes the deactivation and desensitisation of the receptor in the hippocampus thus subsequently facilitating the cognitive performances, including short-term memory [ 29 ].
2.2. Cholinergic System
In regulating the cognitive functions, the central cholinergic system is suggested to be an essential neurotransmitter associated with, namely, acetylcholine (ACh) [ 30 ]. The neuronal nicotinic ACh receptor is established located on the presynaptic terminal and applies an action on hippocampal synaptic transmission via stimulating the release of glutamate [ 31 ]. The activation of nicotinic ACh receptor is stimulated by activation of protein kinase C (PKC). These events subsequently maintain the phosphorylation of the receptor [ 32 ] and sustain the upregulation of glutamate release. Afterward, the high expression of glutamate initiates the long-lasting acceleration of hippocampal synaptic transmission [ 33 ]. Taking piracetam as an example, nootropics are suggested to involve the biochemical modifications in the aged brain [ 34 ]. Treatment of nootropics shows pronounced effects in the impaired brain functions induced by number of noxious stimuli, for example, hypoxia, aging, and injury [ 35 ].
Cognitive dysfunction is related to diminish cholinergic function, treated by stimulation of central cholinergic activity expressing the improvement of cognitive performances [ 36 ]. The loss of neuronal cholinergic observed in the hippocampal area is responsible for the major characteristic of Alzheimer's disease. In treating Alzheimer's disease-type senile dementia, central cholinergic system is suggested to be improved. Administration of established nootropics is established to increase the level of ACh and upregulation of receptor binding for cholinergic in the frontal cortex and hippocampus [ 37 ]. Downregulation of noradrenergic function is studied to diminish the behavioural impairment due to degeneration of cholinergic system [ 38 ]. The nootropics activities are observed through the downregulated ACh esterase activity. The reduction subsequently leads to upregulation of ACh expression in the brain. Thus, good agents of nootropics are able to decrease norepinephrine (NE) and elevate the 5-hydroxytryptamine (5-HT) expression observed in the central cortex, hippocampus, and hypothalamus [ 39 ].
2.3. Amyloid Precursor Protein
Dysfunction of the cholinergic system in Alzheimer's disease is also accompanied by the involvement of amyloid protein, specifically amyloid β -protein, and neurofibrillary tangles [ 40 ]. Modulation of processing of cellular component is also influenced by the neuronal transmission and synaptic plasticity. Amyloid precursor protein (APP) is one example of cellular component affected. APP is detected in the membrane of synaptic preparation and leading to the involvement of a fragment of APP in synaptic formation and maintenance [ 41 ]. The consequences of the influence of APP seem to contribute to the memory formation [ 42 ]. Introduction of natural nootropics increases the learning and memory performance, which causes upregulation of APP expression [ 43 ]. Knockout APP in mice was observed to impair the behavioural activity and alter the structure and length of the neuron [ 44 ]. In introducing natural nootropic, modulation of APP processing must be approached since it mediates the formation of specific neurotropic APP fragments, which is important for memory functions.
Patients diagnosed with Alzheimer's disease are expressed with the deposition of insoluble or oxidised amyloid- β derived from APP present in the brain [ 45 ]. β -amyloid peptide is another fragment derived from the APP, contributing to the impairment of short-term working memory [ 46 ]. Oversynthesis of β -amyloid from APP may be influenced by the increased neuronal activity thus subsequently causing the depression of synaptic transmission [ 47 ]. The patient's brain also contains an activated caspase-3. Caspase-3 is a cysteine protease that facilitates the apoptosis induced by the mitochondrion [ 48 ]. Marx [ 49 ] has listed a possible reason of the onset of Alzheimer's disease, namely, due to deposition of amyloid- β , apoptosis, and presence of oxidative stress. The amyloid- β -induced apoptosis leads to the neuronal degeneration [ 50 ]. High expression of amyloid- β in the neuron stimulates neuronal apoptosis death due to induction of caspase-3 activities [ 51 ]. Production of amyloid- β fibril is an indicator for development of Alzheimer's disease. The amyloid- β fibril is responsible for permeability of lipid membrane [ 52 ] and stimulation of Ca 2+ conductance [ 53 ].
2.4. Secondary Messenger
Schwartz [ 54 ] has claimed the involvement of secondary messenger implicated in the cognitive purpose. The evolution of the intracellular signalling cascade involves various enzymes and selective protein-protein interactions in response to the cognitive performance. LTP, as mentioned before, is related to the activation of NMDA receptor and leads to influx of Ca 2+ . It is originating the series of events causing the activation of pre- and postsynaptic mechanisms [ 55 ]. Ca 2+ is observed to activate PKC in the dentate gyrus [ 56 ], a molecule that is involved in learning and memory processes [ 57 ]. The administration of PKC activator [ 58 ] and nootropic drugs were observed to improve the memory performances, suggesting the involvement of similar pathway, the PKC pathway [ 59 ].
Upon PKC activation, it localises to specific subcellular sites and confers different physiological function [ 60 ]. The failure for this translocation to occur is found in normal ageing and number of neuronal pathologies [ 61 ]. Considering the role of PKC in learning and memory mechanisms, PKC improves synaptic plasticity in the brain. In addition, diminished calcium/calmodulin-dependent protein kinase II (CaMKII) and PKC activity contribute to downregulation of NMDA receptor, thus reducing the release of glutamate [ 62 ].
2.5. Miscellaneous
Insulin receptor becomes another target for investigating the effect of nootropic in cognitive purposes. Insulin plays a role in the neuropathological views, including involvement in neurotropic and neuromodulatory functions [ 63 ]. In the central nervous system (CNS), insulin is synthesised and released from neuron as a response to depolarisation [ 64 ]. As observed from a number of studies, insulin receptors are also responsible for learning and memory [ 65 ]. High expression of insulin receptors is found in the hippocampus, including in the dentate gyrus and CA1 pyramidal cells [ 66 ]. Neuron insulin or insulin receptor in the hippocampus can modulate the synaptic activity mediated by NMDA, which subsequently suppress the AMPA and GABA A receptors. It alters the synthesis and the activity of a number of neurotransmitters [ 67 ]. Activation of neuronal insulin will stimulate the signalling pathway of cognitive function, including activation of mitogen-activated protein kinase (MAPK), PKC, and phosphatidylinositol-3-kinase (PI3K) [ 67 ]. Diabetic rats were observed to experience cognitive impairment and LTP [ 68 ].
Other than insulin receptor, angiotensin receptor also facilitates the signal transduction in the CNS. Angiotensin, a peptide hormone, is part of the renin-angiotensin system that stimulates the release of aldosterone from the adrenal cortex. Angiotensin II (Ang II), one of the subtypes of angiotensin, regulates the blood pressure via a number of actions. The most significant actions are vasoconstriction, renal actions, and increased aldosterone biosynthesis [ 69 ]. Ang II also interacts with other neurotransmitters, causing the release of noradrenaline and the synthesis of serotonin [ 70 ]. Ang II facilitates the cognitive and behavioural processes through its specific receptors and metabolites expressed in animal models [ 71 ]. Upon cognitive impairment of Alzheimer's disease patient, the Ang II in the brain inhibits the release of acetylcholine through inhibition on angiotensin type 1 receptor (AT1) [ 72 ]. Acetylcholine is known as a critical neurotransmitter responsible for memory.
Review by van der Staay et al. [ 73 ] has established a definition of cognition enhancer. Overall, a cognition enhancer is a pharmacological compound that enhances the mnemonic and cognitive function that can cross the blood-brain barrier. The cognition function is influenced by the cognition enhancer including the learning, consolidation and retrieval, and memory. As a contrast, it does not have psychopharmacological activity such as sedation and has minimal or no adverse effects with low toxicity level. Listed below are examples of natural nootropic that were proved to improve cognitive and memory properties.
3. Examples of Nootropic
3.1. pyrrolidinone derivatives.
Pyrrolidinone is a class of 5-membered lactams with a four-carbon heterocyclic ring structure with biological interest [ 74 ] found in many pharmaceuticals and natural products. The synthesis of nootropic from pyrrolidinone derivatives has common features including enhancing the learning process, diminishing the impaired cognition, and protecting against brain damage. Number of pyrrolidine derivatives are commercially available, including piracetam, oxiracetam, aniracetam, and promiracetam [ 75 ]. Administration of aniracetam or piracetam affects the muscarinic receptor binding in the different brain regions [ 76 ]. Study by Pilch and Müller [ 77 ] had established the upregulation of m-cholinoceptor in the brain responding to the aging brain.
Piracetam or 2-oxo-1-pyrrolidineacetamide, a cyclic derivative of gamma-aminobutyric acid (GABA) [ 78 ], is widely used in treating senile dementia and Alzheimer's disease [ 79 ]. Studies showed the role of piracetam in enhancing the memory and learning [ 80 ] and act synergistically with choline leading to greater enhancement of cognition. Winnicka et al.'s study [ 78 ] showed the effect of piracetam on regulating the release of glutamate observed in the cortex and hippocampus, suggesting involvement of NMDA receptor induced by piracetam. Despite having a low affinity for glutamate receptors, piracetam initiates a number of effects on the receptors, for example, on AMPA receptor. Treatment of piracetam causes activation of AMPA receptor thus stimulating the influx of Ca 2+ in the brain and increasing the density of AMPA receptors in the synaptic membrane of the cortex. Piracetam also causes the release of glutamate stimulated by potassium in the hippocampal nerves [ 81 ]. Recent reports suggest the neuroenhancing effect of piracetam is via stimulation of acetylcholinergic and glutamatergic systems, plus elevation of membrane permeability [ 82 ].
Introduction of aniracetam is usually related to the involvement of AMPA receptor [ 83 ], cholinergic system [ 84 ], and metabotropic receptor [ 85 ], as part of cognition function. Aniracetam, including pyrrolidinone derivative compounds, is established to diminish the cognitive impairment [ 86 ]. Systemic administration of aniracetam improves the cognitive performance observed behaviours, suggesting the involvement of AMPA in the dentate gyrus [ 87 ]. The effects of cognitive enhancer of aniracetam are postulated due to the slow rate of deactivation [ 88 ] and desensitisation of AMPA receptors [ 89 ] observed using hippocampal slide. Other studies had suggested the involvement of activated hippocampal PKC and sustained ratio of membrane and cytosolic PKC γ [ 90 ]. The enhancement of PKC γ is subsequently induced by the phosphorylation of glutamate receptor subunits, thus modifying the channel kinetics of AMPA receptor [ 91 ]. The study showed that the intrahippocampal aniracetam mediates the formation of behavioural LTP, thus representing the synaptic mechanism induced by the treatment [ 83 ].
Another example of pyrrolidinone derivative is nefiracetam (N-(2,6-dimethyl-phenyl)-2(2-oxo-1-pyrrolidinyl)), a piracetam-like nootropic agent. Studies show that the compound improves the impaired cognitive due to drugs [ 92 ], morphine [ 93 ], or ageing [ 94 ]. Intake of nefiracetam is postulating the cholinergic system, as ACh receptor enhances the release of neurotransmitter [ 31 ]. Nefiracetam is studied to influence the phosphorylation of nicotinic ACh receptor by activating the PKC, thus helping the release of neurotransmitter from the presynaptic terminal [ 32 ]. Synaptic transmission influenced by nefiracetam is not mediated through the blocking of GABAergic transmission and enhanced postsynaptic ionotropic glutamate receptor. Interestingly, nefiracetam is improving the synaptic strength by aiming at the nicotinic ACh receptor [ 33 ] possibly by Na + without affecting the permeability of Ca 2+ [ 32 ]. Another study suggests that the inhibition of PKA is responsible for the effect of nefiracetam on Ca 2+ channel [ 32 ]. In contrast to piracetam and oxiracetam, nefiracetam enhances the N- and L-type Ca 2+ channels but not T-type [ 95 ].
DM235 or sunifiram is a recent compound structurally related to piracetam and is known to prevent cognitive deficits. The compound is observed to improve the impaired cognitive functions by inhibiting the induction of amnesia [ 96 ]. As discussed previously, pyrrolidinone derivatives prevent the amnesia induced by the impaired cholinergic system [ 97 ] and ameliorate the cognitive deficits [ 98 ]. Similar to other pyrrolidinone derivatives, sunifiram increases the release of neurotransmitter from the presynaptic terminal [ 99 ]. Amnesia can be induced by altering the neurotransmitter system through GABA. Activation of GABA receptor impairs the cognitive function including the learning and memory processes [ 100 ]. In contrast to other pyrrolidinone derivatives, sunifiram is more potent while having a similar characteristics with piracetam. Sunifiram is observed to ameliorate the memory function and has less adverse effects [ 81 ]. A recent study reports the improvement of hippocampal LTP induced by sunifiram is mediated by the glycine-binding site of NMDA receptor [ 101 ], an attractive binding site for Alzheimer's disease drugs [ 102 ]. Thus, the reaction of sunifiram on the same binding site is suggested to ameliorate the impaired cognitive function of Alzheimer's disease patients [ 101 ]. Stimulation of the binding is also associated with the increased autophosphorylated CaMKII and PKC α thus suggesting the enhanced memory and hippocampal LTP [ 103 ].
3.2. Bacopa monnieri
Bacopa monnieri or Brahmi is derived from the family of Scrophulariaceae, found throughout the Indian subcontinent in a wet, damp, and marshy area [ 104 ]. It has purple flowers with numerous branches and small oblong leaves ( Figure 1 ). This plant is known to be used for number of nervous system disorders, including insomnia, anxiety, and epilepsy. According to Ayurvedic medical practitioners, Bacopa monnieri is categorised as a medhya rasayana, a compound that stimulates and enhances the memory and intellect. These properties were studied preclinically and clinically [ 105 ]. The property of memory, facilitating action of this plant, is contributed by the chemical constituents of bacoside A, assigned as 3-(a- l -arabinopyranosyl)-O- β - d -glucopyranoside-10, 20-dihydroxy-16-keto-dammar-24-ene [ 106 ], and bacoside B [ 107 ]. The treatment of a mixture of bacosides A and B is mediating the three types of learning function, procedural, declarative, and spontaneous, and improves the episodic memory observed in animal models [ 108 ]. Beside enhancing cognition and memory functions, Bacopa monnieri are also known for their anxiolytic effects and in managing the convulsive sicknesses [ 109 ].

Bacopa monnieri . The plant has purple flowers with oblong leaves found throughout the Indian subcontinent. This plant is classified under the family of Scrophulariaceae. On the right is the chemical structure for bacosides [ 107 ].
Singh and colleagues [ 110 ] had suggested the membrane dephosphorylation triggered by bacosides concurrently leads to elevation in protein and RNA turnover observed in certain brain regions. The nootropics effect of Bacopa monnieri is mediated by enhancement of protein kinase activity and production of protein in the hippocampus [ 39 ]. Study done by Anand and colleagues [ 111 ] demonstrated the characteristics of natural antioxidant and DNA damage preventing agent of the Bacopa monnieri . Other effects of Bacopa monnieri are including hepatoprotective agent against morphine toxicity [ 112 ], calcium antagonist [ 113 ], anticancer agent [ 114 ], and antiaddictive agent [ 115 ]. Despite that, the combination of bacosides A and B also was studied to express antistress property [ 116 ], protecting the brain from smoking induced membrane damage [ 117 ], and protective function against d -galactosamine induced liver injury [ 118 ].
3.3. Nicotine
Nicotine is a potent parasympathomimetic alkaloid derived from the family of plants of Solanaceae ( Figure 2 ). The psychoactive nicotine is found in the leaves of Nicotiana rustica , the tobacco plant Nicotiana tabacum , Duboisia hopwoodii , and Asclepias syriaca [ 119 ]. Despite its addiction liability and undesired adverse effects [ 120 ], nicotine is found to improve learning and memory properties and enhance the memory impairment due to lesion of the septohippocampal pathways or aging. Downregulated expression of nicotinic receptor is observed in Alzheimer's disease patients [ 121 ].

Nicotiana mutabilis . The plant is classified under the family of Solanaceae and contains nicotine as psychoactive compound (a). Compound A, syn-5-isobutoxy-2-phenyl-3-(3-pyridyl)-isoxazolidine (b), and compound B, syn-2,5-diphenyl-3-(3-pyridyl)-isoxazolidine (c), are example of synthesised nootropics derived from nicotine for learning and memory purposes [ 122 ].
Due to the prohibition of the use of nicotine, novel nicotine analogue is synthesised and evaluated, namely, syn-5-isobutoxy-2-phenyl-3-(3-pyridyl)-isoxazolidine (compound A) and syn-2,5-diphenyl-3-(3-pyridyl)-isoxazolidine (compound B) [ 122 ]. Nicotine and its synthesised analogs are established to react on different pathways, expressing improvement in memory [ 123 ]. Compounds A and B are postulated to stimulate the release of acetylcholine through the activation of presynaptic nicotine acetylcholine receptors. These receptors are responsible for modulating the release of neurotransmitter [ 124 ].
3.4. Ginkgo biloba
Ginkgo biloba or maidenhair tree is the only species derived from family of Ginkgophyta and the order of Ginkgoales. It is called a “living fossil” since the morphology and features of the plant are changed for over 100 million years [ 125 ]. The plant is well-known for its medical used as well as being a source of food [ 126 ]. Despite the lack of reports, Ginkgo biloba is claimed to have neuroprotective effects observed in human and animal models [ 127 ]. A recent report has suggested the effect of Ginkgo biloba in treating Alzheimer's disease patient or other cognitive disoders. Ginkgo biloba also has been listed under group of antidementia drugs [ 128 ]. It acts as antioxidant and antiapoptotic properties and also induces inhibition effects against caspase-3 activation and amyloid- β -aggregation toward Alzheimer's disease.
The extract of the leaves diminishes the amyloid- β fibrillogenesis, reduces the apoptosis induced by mitochondria, and downregulates the caspase-3 activity [ 51 ]. This plant also is proposed to have antiamyloidogenic property whereby the plant extract prevents the production of amyloid fibrils [ 51 ]. The compound found in Ginkgo biloba , terpenoid, namely, bilobalide and ginkgolide, is observed to be involved in the caspase-3 activation [ 51 ]. Nakanishi [ 125 ] has proposed the memory enhancing effect of ginkgolides. The ginkgolides are compounds in the plant that terminate the effects of amyloid peptide on LTP ( Figure 3 ).

Ginkgo biloba . The plant is classified under the family of Ginkgoaceae, the only species in the division of Ginkgophyta. With 40 m in height, this tree is characterised by the fan-shaped leaves composed of more than two distinct lobes (a). Ginkgolide A, R 1 =H; R 2 =H; R 3 =OH, Ginkgolide B, R 1 =OH; R 2 =H; R 3 =OH, Ginkgolide C, R 1 =OH; R 2 =OH; R 3 =OH, Ginkgolide J, R 1 =H; R 2 =OH; R 3 =OH, Ginkgolide M, R 1 =OH; R 2 =OH; R 3 =H (b) [ 129 ].
3.5. Panax ginseng
Panax ginseng (Asian ginseng) is described as the “king herb" and has an important position in the traditional Chinese medicine [ 130 ]. A lot of reports are discussing the role of P. ginseng especially in improving the cognition function of Alzheimer's disease patients. Antioxidant property in P. ginseng is claimed to suppress Alzheimer's disease-like pathology [ 131 ]. The intake of P. ginseng in healthy individuals is observed to increase the memory performances [ 132 ].
The active constituents of the Panax spp. are ginsenoside saponins, which are divided into Panaxadiol, Panaxatriol, and oleanolic acid groups. The Panaxadiol and Panaxatriol groups are studied to increase the release of neurotransmitters in the brain [ 133 ]. Other ginsenosides affect the secretion of corticosterone and uptake of NE, dopamine, serotonin, and GABA [ 134 ]. It is suggested that the high ratio of Panaxatriol to Panaxadiol is responsible for the enhancement of memory and cognitive properties [ 135 ]. P. quinquefolius (American ginseng) has a lower ratio of Panaxatriol to Panaxadiol as compared to P. ginseng (Asian ginseng) ( Figure 4 ) [ 136 ].

Panax ginseng . Ginseng belongs to the genus Panax of the family Araliaceae, found in the cooler climates. The name of the plant is derived from the Chinese term meaning “person” and “plant root” due to the feature of the root that resembles the legs of a person (a). Ginsenosides, the principal bioactive compounds of P. ginseng . A, Panaxadiol; B, Panaxatriol; C, oleanolic acid (b) [ 137 ].
3.6. Rhodiola rosea
Rhodiola rosea ( R. rosea ), known as golden root and Arctic root, is reported to improve cognitive function [ 138 ], enhance memory and learning [ 139 ], and protect the brain [ 140 ]. Belonging to the family of Crassulaceae, this plant is observed to increase the level of 5-HT and NE in the cerebral, prefrontal, and frontal cortex [ 139 ]. At the same time, the intake of R. rosea causes the upregulation of DA and ACh in the limbic system pathways, responsible for emotional calming [ 141 ], as R. rosea is acting as antioxidant agent. The study showed that the introduction of R. rosea may protect the nervous system against oxidative damage, thus lowering the risk of Alzheimer's disease onset. The treatment of the plant also enhances the learning and memory impairment in Alzheimer's disease [ 142 ]. Sharing the same property with Bacopa monnieri and Panax ginseng , R. rosea is considered to be an “adaptogen” that enhances endurance, resistance, and protest against stressful situation [ 143 ]. Salidroside, an active component of R. rosea , is claimed to have neuroprotective and antioxidative effects ( Figure 5 ) [ 140 ].

Rhodiola rosea . It belongs to the family of Crassulaceae. R. rosea is growing on the sea cliffs and on the mountains. The plant is dioecious, with yellow to greenish yellow flowers (a). Salidroside is claimed as an active constituent responsible for neuroprotective and antioxidant properties (b) [ 140 ].
4. Conclusion
The understanding of the mechanisms influenced by the administration of natural nootropics has been expanded tremendously in this past decade. Establishing natural nootropic is challenging as optimum dose has to pass blood brain barrier so that it can stimulate responding mechanism. In the same time, the nootropic is helping the body systems such as blood circulation as well as energy booster. There are a number of mechanisms influenced by the administration of nootropics, such as glutaminergic signalling and amyloid precursor protein, also responsible for neuro-related diseases such as dementia and Alzheimer's disease. Thus, the understanding of the mechanism stimulated by nootropic is expected to increase the cognitive performances of the cognitive impairment patients.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Nootropics, or ‘Smart Drugs,’ Are Gaining Popularity. But Should You Take Them?
T he idea that a pill can supersize human intelligence is decidedly science fiction. But plenty of real-world researchers and drug-makers are working to develop nootropics: pills, supplements and other substances designed to improve various aspects of cognition.
A rough translation for the word “nootropic” comes from the Greek for “to bend or shape the mind.” And already, there are dozens of over-the-counter (OTC) products—many of which are sold widely online or in stores—that claim to boost creativity, memory, decision-making or other high-level brain functions. Some of the most popular supplements are a mixture of food-derived vitamins, lipids, phytochemicals and antioxidants that studies have linked to healthy brain function. One popular pick on Amazon, for example, is an encapsulated cocktail of omega-3s, B vitamins and plant-derived compounds that its maker claims can improve memory, concentration and focus.
But do “smart pills” really work?
Many of these supplements include exotic-sounding ingredients. Ginseng root and an herb called bacopa are two that have shown some promising memory and attention benefits, says Dr. Guillaume Fond, a psychiatrist with France’s Aix-Marseille University Medical School who has studied smart drugs and cognitive enhancement. “However, data are still lacking to definitely confirm their efficacy,” he adds.
Many of the food-derived ingredients that are often included in nootropics— omega-3s in particular , but also flavonoids —do seem to improve brain health and function. But while eating fatty fish, berries and other healthy foods that are high in these nutrients appears to be good for your brain, the evidence backing the cognitive benefits of OTC supplements that contain these and other nutrients is weak.
A 2015 review of various nutrients and dietary supplements found no convincing evidence of improvements in cognitive performance. While there are “plausible mechanisms” linking these and other food-sourced nutrients to better brain function, “supplements cannot replicate the complexity of natural food and provide all its potential benefits,” says Dr. David Hogan, author of that review and a professor of medicine at the University of Calgary in Canada.
Even if you eat foods that contain these nutrients, Hogan says their beneficial effects are in many ways cumulative—meaning the brain perks don’t emerge unless you’ve been eating them for long periods of time. Swallowing more of these brain-enhancing compounds at or after middle-age “may be beyond the critical period” when they’re able to confer cognitive enhancements, he says.
None of this rules out the potential for some OTC nootropics to improve memory, focus or other aspects of cognition. There just isn’t much compelling evidence to support these claims.
Certain pharmaceuticals could also qualify as nootropics. For at least the past 20 years, a lot of people—students, especially—have turned to attention deficit hyperactivity disorder (ADHD) drugs like Ritalin and Adderall for their supposed concentration-strengthening effects. While there’s some evidence that these stimulants can improve focus in people without ADHD, they have also been linked, in both people with and without an ADHD diagnosis, to insomnia, hallucinations, seizures, heart trouble and sudden death, according to a 2012 review of the research in the journal Brain and Behavior . They’re also addictive.
More recently, the drug modafinil (brand name: Provigil) has become the brain-booster of choice for a growing number of Americans. According to the FDA , modafinil is intended to bolster “wakefulness” in people with narcolepsy, obstructive sleep apnea or shift work disorder. But when people without those conditions take it, it has been linked with improvements in alertness, energy, focus and decision-making. A 2017 study found evidence that modafinil may enhance some aspects of brain connectivity, which could explain these benefits.
Modafinil has some vocal fans, including the “biohacker” and Bulletproof founder Dave Asprey. And at least some of the existing research on modafinil hasn’t turned up serious long-term health risks.
But there are some potential side effects, including headaches, anxiety and insomnia. Part of the way modafinil works is by shifting the brain’s levels of norepinephrine, dopamine, serotonin and other neurotransmitters; it’s not clear what effects these shifts may have on a person’s health in the long run, and some research on young people who use modafinil has found changes in brain plasticity that are associated with poorer cognitive function.
There are some other promising prescription drugs that may have performance-related effects on the brain. But at this point, all of them seem to involve a roll of the dice. You may experience a short-term brain boost, but you could also end up harming your brain (or some other aspect of your health) in the long run. “To date, there is no safe drug that may increase cognition in healthy adults,” Fond says of ADHD drugs, modafinil and other prescription nootropics.
That’s not to say all smart drugs are risky or ineffective.
“One of my favorites is 1, 3, 7-trimethylxanthine,” says Dr. Mark Moyad, director of preventive and alternative medicine at the University of Michigan. He says this chemical boosts many aspects of cognition by improving alertness. It’s also associated with some memory benefits. “Of course,” Moyad says, “1, 3, 7-trimethylxanthine goes by another name—caffeine.”
While caffeine was once considered risky, most experts today agree that caffeine (at least in coffee and green tea) is more beneficial than harmful when it’s consumed in moderation.
But if you’re really looking to boost your brain function in a way that is absolutely, 100% proven to be safe and effective, Moyad says the answer is clear: get some exercise. “I think exercise has the ability to beat any pill for people trying to improve their cognition or memory who are otherwise healthy,” he says.
There’s strong evidence that regular exercise improves memory and fends off age-related cognitive decline. Working out also improves your heart health and lowers your risk for death and disease, Moyad says.
Exercise isn’t as effortless as taking a pill. But it’s likely the safest way to strengthen your brain—at least for now.
More Must-Reads from TIME
- The New Face of Doctor Who
- Putin’s Enemies Are Struggling to Unite
- Women Say They Were Pressured Into Long-Term Birth Control
- Scientists Are Finding Out Just How Toxic Your Stuff Is
- Boredom Makes Us Human
- John Mulaney Has What Late Night Needs
- The 100 Most Influential People of 2024
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at [email protected]
Smart drugs. – Mgr. Hrozek Hrozek
Your browser internet explorer is out of date..
It has known security flaws and may not display all features of this and other websites.
Mgr. Hrozek Hrozek
Master's thesis, smart drugs., thesis defence.
- Supervisor: doc. PharmDr. Jan Gajdziok, Ph.D.
- Reader: doc. PharmDr. Ruta Masteiková, CSc.
Citation record
Iso 690-compliant citation record:, full text of thesis, contents of on-line thesis archive, other ways of accessing the text, masaryk university.
Master programme / field: Pharmacy / Pharmacy
Theses on a related topic
- Elektrochemické chování komplexů železa v roztocích a na povrchu modifikovaných elektrod Jakub Věžník
- Optické biosenzory pro studium polymerních nosičů pro transport léčiv Lucie Peštová
- Hyaluronan based drug carrier systems Kristina Nešporová
- Bacterial components as nanocarriers for drug delivery and their usage in animal and human health care Martin Řiháček
- Vybrané deriváty pyridinu imobilizované na koloidních nanosystémech určených pro asymetrickou katalýzu a pro transport léčiv Dattatry Shivajirao Bhosale
- Synthesis of peptide and their application for cell penetration and drug delivery Vedran Milosavljević
- Vliv struktury polymerních nosičů léčiv na jejich Daniela Kubátová
- Použití polymerních materiálů pro lékové formy na bázi osmotické pumpy Tereza Sklenářová

IMAGES
VIDEO
COMMENTS
Your Personalized Thesis Journey. 1. Take the Quiz. Tell us about yourself and your goals. We'll use your answers to determine your baseline and build your recommendations. 2. Get Your Starter Kit. You'll sample 4 blends over the course of the month to understand what you can accomplish with each formulation. 3.
2. What Are Nootropics? Nootropics, also known as "smart drugs" in English language journals [], are a heterogeneous group of compounds [].The term "nootropic" was first used by Cornelius E. Giurgea in 1972/1973 [10,11] to describe substances that primarily activate cognitive functions, such as memory and learning, especially in situations where these functions are impaired [].
'Smart drugs' (also known as 'nootropics' and 'cognitive enhancers' [CEs]) are being used by healthy subjects (i.e. students and workers) typically to improve memory, attention, learning, executive functions and vigilance, hence the reference to a 'pharmaceutical cognitive doping behaviour'. While the efficacy of known CEs in individuals with memory or learning deficits is well ...
Design rationale of smart drug delivery nanoplatforms. It is well known that drugs should ideally be released at the target sites in a controlled manner to enhance their therapeutic efficiency, meanwhile, to reduce the side effects. Inheriting from the controlled release nanoplatforms, the loaded drugs can act "smart".
Introduction: Cognitive enhancers (CEs), also known as "smart drugs", "study aids" or "nootropics" are a cause of concern. Recent research studies investigated the use of CEs being taken as study aids by university students. This manuscript provides an overview of popular CEs, focusing on a range of drugs/substances (e.g ...
Forty years after the first reports on stimuli-responsive phase transitions in synthetic hydrogels, the first medicines based on responsive components are approaching the market. Sensitiveness to internal or external signals of the body can be achieved by means of materials (mostly polymers, but also lipids
The use of drugs by people hoping to boost mental performance is rising worldwide, finds the largest ever study of the trend. In a survey of tens of thousands of people, 14% reported using ...
Numerous Internet-based interventions, such as mobile phone applications providing disease and medication information, electronic reminders via mobile phone text messages or emails, electronic pill boxes and web-based systems for medication monitoring and education, among others, are being developed and used to address medication management, with the goal of improving medication adherence. 7-9 ...
Nootropics, also known as "smart drugs" are a diverse group of medicinal substances whose action improves human thinking, learning, and memory, especially in cases where these functions are impaired. This review provides an up-to-date overview of the potential effectiveness and importance of nootropics. Based on their nature and their effects ...
Smart drugs were allegedly circulating, helping players to get in the zone. "Just like in normal sports, it's not OK to win because you took a pill," says Anna Rozwandowicz, ESL's director of ...
The current study explores the concept of Molecular Imprinting Technology (MIT) and evaluates the ability of a molecularly imprinted hydrogel polymer (MIP) to preferentially uptake the template drug propranolol from aqueous solution. The extent of the molecular affinity and recognition was challenged by introducing a secondary competing structure during uptake. The release of propranolol as a ...
Smart drugs:Improving healthcare using Smart Pill Box for Medicine Reminder and Monitoring System. Author links open overlay panel Diaa Salama Abdul Minaam, Mohamed Abd-ELfattah. Show more. ... M.S.thesis. Google Scholar [18] S.-C. Huang, H.-Y. Chang,Y.-C. Jhu and G.-Y. Chen, "The intelligent pill box-design and implementation," in ...
Nanoparticles-based drug delivery research that led to a promising and viable technology platform has been accepted as an alternative drug delivery system to reduce limitations of the conventional chemotherapy. Recently, inorganic biodegradable pH-sensitive carbonate apatite (CA), a potential drug nanocarrier have been shown to confer great advantages to carry the anti-cancer drugs as well as ...
Nootropics, or smart drugs, aim to enhance cognitive performance. Prescription stimulants and nonprescription substances, including caffeine, are considered nootropic. Here, we discuss research ...
The "nootropic" or simplified as a "smart drug," "brain booster," or "memory enhancing drug," is a common term that will tag along with the compound responsible for the enhancement of mental performance. By definition, nootropic is a compound that increases mental functions including memory, motivation, concentration, and ...
The term "nootropics" first referred to chemicals that met very specific criteria. But now it's used to refer to any natural or synthetic substance that may have a positive impact on mental skills ...
DOI: 10.1016/J.FCIJ.2018.11.008 Corpus ID: 69553243; Smart drugs:Improving healthcare using Smart Pill Box for Medicine Reminder and Monitoring System @article{AbdulMinaam2018SmartDH, title={Smart drugs:Improving healthcare using Smart Pill Box for Medicine Reminder and Monitoring System}, author={Diaa Salama Abdul Minaam and Mohamed Abdelfattah}, journal={Future Computing and Informatics ...
That's not to say all smart drugs are risky or ineffective. "One of my favorites is 1, 3, 7-trimethylxanthine," says Dr. Mark Moyad, director of preventive and alternative medicine at the ...
Nootropics ( / noʊ.əˈtroʊpɪks / noh-ə-TROHP-iks or / noʊ.əˈtrɒpɪks / noh-ə-TROP-iks; [1] but not / njuːˈtroʊpɪks / new-TROHP-iks or / njuːˈtrɒpɪks / new-TROP-iks, [1] which are common mispronunciations), colloquially brain supplements, smart drugs and cognitive enhancers, are natural, semisynthetic or synthetic compounds ...
Rhodiola Rosea. The herb Rhodiola rosea is an adaptogen (herbs, plants or mushrooms that help the body respond to stress) promoted to improve energy, balance mood and enhance brain function, says ...
This function allows them to control, modify and aim the release of drugs from their application forms. These targeted delivery systems can significantly improve therapy, reduce side effects, and increase patient compliance. The presented thesis provides an overview of smart drug delivery systems and describes their use in therapy on examples.
Based on Take Thesis reviews, the goal of Clarity is to sharpen your focus and clear your brain fog, which could help you think better. It combines natural ingredients - like 7,8-DHF, Alpha-GPC ...