Lung cancer
Affiliations.
- 1 Peter MacCallum Cancer Centre, Melbourne, VIC, Australia; Sir Peter MacCallum Department of Oncology, University of Melbourne, VIC, Australia.
- 2 Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
- 3 Department of Medicine, Massachusetts General Hospital, Boston, MA, USA. Electronic address: [email protected].
- PMID: 34273294
- DOI: 10.1016/S0140-6736(21)00312-3
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable progress in the past two decades and transformed outcomes for many patients. This seminar provides an overview of advances in the screening, diagnosis, and treatment of non-small-cell lung cancer and small-cell lung cancer, with a particular focus on targeted therapies and immune checkpoint inhibitors.
Copyright © 2021 Elsevier Ltd. All rights reserved.

Publication types
- Research Support, Non-U.S. Gov't
- Carcinoma, Non-Small-Cell Lung / diagnosis*
- Carcinoma, Non-Small-Cell Lung / therapy*
- Lung Neoplasms / diagnosis*
- Lung Neoplasms / therapy*
- Small Cell Lung Carcinoma / diagnosis*
- Small Cell Lung Carcinoma / therapy*
The role of imaging in the diagnosis of lung cancer in primary care
--> Bradley, Stephen (2022) The role of imaging in the diagnosis of lung cancer in primary care. PhD thesis, University of Leeds.
Background Lung cancer is the leading cause of cancer death worldwide. The UK relies more heavily upon chest x-ray than many other high income countries. Little is known about the performance of the test, the risk of cancer following negative test, consequences of ‘false negative’ results and the factors that affect how frequently chest x-ray is used. Aims 1. Determine sensitivity and specificity of chest x-ray. 2. Determine if there are differences in outcomes between patients with ‘true positive’ versus ‘false negative’ chest x-rays. 3. Determine the risk of lung cancer following a negative chest x-ray with respect to symptoms. 4. Quantify the volume of chest x-rays undertaken by English general practices and understand the extent to which variations in chest x-ray frequency are due to differences in patient populations and the practices themselves. Methods 1. Systematic review on sensitivity of chest x-ray 2. Observational study to determine sensitivity and compare stage and survival between those with ‘true positive’ versus ‘false negative’ results. 3. Cohort study to determine chest x-ray specificity and lung cancer risk following negative chest x-ray. 4. Retrospective study to quantify general practices’ chest x-rays with respect to characteristics of their patient populations and the practices. Results 1. Sensitivity was 77-80% (systematic review) and 82% (observational study). Specificity was 90%. 2. ‘False negative’ chest x-rays were not associated with adverse outcomes, although given the retrospective methodology this cannot be excluded. 3. Lung cancer risk following negative chest x-ray was <1% for all symptoms except haemoptysis (3%). 4. There was substantial variation in chest x-ray utilisation (median 34/1000 patients, IQR 26-43), with 18% of variance accounted for by recorded characteristics. Conclusions Chest x-ray does not identify ~20% of lung cancers but it continues to have a useful role. The substantial variation in rates of investigation suggest that it may be underutilised in many practices.
--> Final eThesis - complete (pdf) -->
Filename: Thesis 04 05 22unmarked.pdf

Embargo Date:
You do not need to contact us to get a copy of this thesis. Please use the 'Download' link(s) above to get a copy. You can contact us about this thesis . If you need to make a general enquiry, please see the Contact us page.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Immunol
Immunotherapy in Lung Cancer: Current Landscape and Future Directions
Hirva mamdani.
1 Department of Oncology, Barbara Ann Karmanos Cancer Institute, Wayne State University, Detroit, MI, United States
Sandro Matosevic
2 Department of Industrial and Physical Pharmacy, Purdue University, West Lafayette, IN, United States
Ahmed Bilal Khalid
3 Department of Internal Medicine, Indiana University, Indianapolis, IN, United States
Gregory Durm
4 Department of Internal Medicine, Division of Hematology/Oncology, Indiana University Melvin and Bren Simon Cancer Center, Indiana University School of Medicine, Indianapolis, IN, United States
Shadia I. Jalal
Over the past decade, lung cancer treatment has undergone a major paradigm shift. A greater understanding of lung cancer biology has led to the development of many effective targeted therapies as well as of immunotherapy. Immune checkpoint inhibitors (ICIs) have shown tremendous benefit in the treatment of non-small cell lung cancer (NSCLC) and are now being used as first-line therapies in metastatic disease, consolidation therapy following chemoradiation in unresectable locally advanced disease, and adjuvant therapy following surgical resection and chemotherapy in resectable disease. Despite these benefits, predicting who will respond to ICIs has proven to be difficult and there remains a need to discover new predictive immunotherapy biomarkers. Furthermore, resistance to ICIs in lung cancer is frequent either because of a lack of response or disease progression after an initial response. The utility of ICIs in the treatment of small cell lung cancer (SCLC) remains limited to first-line treatment of extensive stage disease in combination with chemotherapy with modest impact on overall survival. It is thus important to explore and exploit additional targets to reap the full benefits of immunotherapy in the treatment of lung cancer. Here, we will summarize the current state of immunotherapy in lung cancer, discuss novel targets, and explore the intersection between DNA repair defects and immunotherapy.
Introduction
Lung cancer is the number one cause of cancer related mortality. Over the last decade, a greater understanding of the biology of lung cancer at the molecular level has led to the development of new and effective therapies resulting in improvement in overall survival (OS), mostly driven by advances in the treatment of non-small cell lung cancer (NSCLC) ( 1 ). Traditionally, advanced NSCLC was thought to be one disease. More recently, it has been recognized that NSCLC is on a biological level a set of multiple diseases. Targeted treatments are standard of care in driver mutation positive NSCLC with numerous approved targeted therapies ( 2 ). In patients without a driver mutation, immunotherapy in the form of immune checkpoint inhibitor(s) (ICIs) is currently an integral part of the treatment ( 2 ). Programmed cell death ligand-1 (PD-L1) is one of the predictive biomarkers of response to checkpoint inhibitors. PD-L1 expression is an imperfect biomarker but is the most robust clinical one. NSCLC, however, continues to have a high mortality rate. Most patients with advanced disease eventually progress on first-line treatment and second-line options are limited for patients without a targetable mutation. Moreover, the clinical application of ICI in the treatment of small cell lung cancer (SCLC) has significantly lagged behind that of NSCLC. There remains a critical need to further harness the power of the immune system, expand treatment options, and delay resistance to ICIs. In this article, we will discuss the current standard immunotherapy treatments and potential novel immunotherapy targets and approaches in lung cancer. We will, in addition, shed light on the interplay between DNA repair defects and immunotherapy, an area of great research interest.
Current Role of Immunotherapy in Lung Cancer
The relentless nature of any cancer is often attributed to its vast mutational repertoire equipping the cancer cells with mechanisms to develop resistance to commonly used treatment strategies. It is not surprising that lung cancer, with its major histologic subtypes, is among the top five tumor types carrying the highest number of somatic mutations ( 3 ). In the first decade of 21 st century, median OS of patients diagnosed with advanced NSCLC and SCLC was one year. The discovery of actionable driver genomic alterations and development of targeted therapies led to striking improvement in OS of a subset of NSCLC patients. The survival of the vast majority of patients with NSCLC without an actionable genomic driver and virtually all patients with SCLC remained limited and platinum-based chemotherapy was the mainstay of first-line therapy for these patients.
The discovery of immune checkpoints and subsequent development of Nobel Prize winning ICIs have brought in a radical revolution in the therapeutic landscape of lung cancer, specifically NSCLC ( 4 , 5 ). Of the several known immune checkpoints utilized by the tumor to evade host immune system, the best known and farthest along in clinical application is programmed cell death protein-1/programmed cell death ligand-1(PD-1/PD-L1) and cytotoxic T lymphocyte antigen-4 (CTLA-4) pathways. Inhibition of these pathways enables priming and anti-tumor activity of cytotoxic T-cells, the essential steps that are otherwise inhibited by the expression of B7-1/2 and PD-L1 by the antigen presenting cells carrying tumor associated antigens and tumor cells, respectively ( 4 ).
The first breakthrough in the utility of ICIs in treatment of lung cancer was in the form of PD-1 inhibitor nivolumab as second-line therapy for patients with advanced NSCLC, when randomized phase III trials showed superior objective response rate (ORR) and OS with nivolumab compared to docetaxel in patients with advanced squamous and non-squamous NSCLC following progression on platinum-based chemotherapy ( 6 , 7 ). Shortly thereafter, another PD-1 inhibitor pembrolizumab and PD-L1 inhibitor atezolizumab were approved by the US FDA for the same indication, based on superior efficacy of these agents compared to docetaxel in second-line setting ( 8 , 9 ). The success of ICIs in second-line setting paved the way for their use in first-line treatment of advanced NSCLC. A plethora of phase III clinical trials reported over the past five years, showing durable responses and unprecedented improvement in OS with ICI or ICI plus platinum-based chemotherapy compared to chemotherapy alone, have rapidly expanded first-line treatment options for patients with advanced NSCLC not harboring sensitizing EGFR mutations or ALK translocations. These options include pembrolizumab, atezolizumab, cemiplimab, nivolumab plus CTLA-4 inhibitor ipilimumab, pembrolizumab plus platinum-based chemotherapy, atezolizumab plus platinum-based chemotherapy with or without bevacizumab (for non-squamous histology), and nivolumab plus ipilimumab plus two cycles of platinum-based chemotherapy ( 10 – 17 ). The choice of therapy in clinical practice is largely determined by PD-L1 expression, burden of disease, and tumor mutation profile. Besides the improvement in response rates and OS, one of the most fascinating aspects of using ICI-based therapies in NSCLC is the durability of survival benefit. For example, recently reported 5-year outcomes of landmark KEYNOTE-024 trial comparing pembrolizumab with chemotherapy as first-line treatment for patients with advanced NSCLC harboring PD-L1 expression of ≥50% demonstrated unprecedented 5-year OS of 32% with pembrolizumab ( 18 ). Randomized trials comparing nivolumab with docetaxel in second-line treatment of advanced NSCLC have also reported that a subset of patient derive prolonged and clinically meaningful survival benefit with ICI ( 19 ).
The success march of ICI in NSCLC has expanded to unresectable stage III and more recently to resectable stage II-IIIA disease. In a randomized phase III trial comparing PD-L1 inhibitor durvalumab with placebo in patients with unresectable stage III NSCLC who had non-progressive disease following concurrent chemoradiation, durvalumab showed superior progression free survival (PFS) and OS which were sustained at 5-year follow up, further affirming the durability of anti-tumor activity of ICI in NSCLC ( 20 , 21 ). Another phase III trial comparing atezolizumab with best supportive care in patients with resectable stable IB-IIIA NSCLC following complete surgical resection and adjuvant platinum-based chemotherapy showed superior disease-free survival (DFS) with atezolizumab which led to recent FDA approval of the agent for adjuvant therapy for patients with resected stage II-IIIA disease with positive PD-L1 expression ( 22 ).
In contrast to the exponential growth of the ICI field in NSCLC, the success in SCLC remains disappointing. The only breakthrough in the treatment of extensive-stage SCLC over the past three decades has been the addition of PD-L1 inhibitors durvalumab or atezolizumab to platinum-based chemotherapy for first-line treatment. While the combination has now become the standard of care, the improvement in median OS with the addition of ICI to chemotherapy is modest at best ( 23 , 24 ). The utility of ICI therapy, either concurrently or sequentially following chemoradiation, in patients with limited-stage SCLC is being evaluated in clinical trials (ClinicalTrials.gov identifiers: {"type":"clinical-trial","attrs":{"text":"NCT03811002","term_id":"NCT03811002"}} NCT03811002 , {"type":"clinical-trial","attrs":{"text":"NCT03703297","term_id":"NCT03703297"}} NCT03703297 ), although a recently reported randomized phase II trial showed no improvement in PFS with nivolumab and ipilimumab following chemoradiation in this setting ( 25 ).
Biomarkers of Response and Resistance to Immunotherapy in Lung Cancer
Despite the increasing role of immunotherapy and specifically PD-1/PD-L1 checkpoint inhibition in lung cancer, a substantial number of patients do not benefit from these therapies. In addition, immune-related toxicities occur in a subset of patients diminishing quality of life, adding to healthcare costs, and resulting in severe impairment or death ( 26 , 27 ). Considering this, it is imperative that clinically useful predictive biomarkers be developed to appropriately choose those patients most likely to benefit and to avoid those with a small chance of efficacy and/or increased risk of toxicity.
PD-L1 Expression
With the exception of genomic driver mutations, PD-L1 is the only biomarker recommended by the National Comprehensive Cancer Network (NCCN) guidelines to aid in making treatment decisions in metastatic NSCLC ( 2 ). Multiple studies have demonstrated the predictive capability of this biomarker in stage IV NSCLC ( 8 , 10 , 28 ). Nearly all these trials have suggested not only that PD-L1 expression levels can help with patient selection, but also, that the degree of immunotherapy benefit can be predicted by the magnitude of PD-L1 expression ( 9 , 14 , 29 , 30 ). In contrast, there are several trials which dispute the benefit of PD-L1 as a viable biomarker for predicting response to PD-1/PD-L1 checkpoint inhibitors ( 6 , 7 , 31 ).
The predictive role of PD-L1 expression in the non-metastatic setting is gradually transpiring. In PACIFIC trial, PD-L1 negative patients exhibited less robust PFS and OS compared to PD-L1 positive patients following treatment with durvalumab ( 32 ). In contrast to this, correlative analysis of a smaller phase II trial of consolidation pembrolizumab after chemoradiation showed no difference in outcomes for PD-L1 positive versus negative patients ( 33 ). In the adjuvant setting, DFS was improved with atezolizumab following surgery and adjuvant chemotherapy only in the PDL1≥1% group, with the PD-L1 ≥50% group exhibiting the largest benefit ( 22 ).
Tumor Mutational Burden (TMB)
TMB is defined as the total number of mutations per megabase (Mb) of exonic regions of evaluated genes in a tumor specimen. As the number of mutations increases, the potential number of new transcribed proteins and neoantigens also goes up. This increase in neoantigen presence is hypothesized to enhance tumor immunogenicity and improve the likelihood that patients respond to checkpoint inhibition. Tissue and blood based TMB have been studied in several lung cancer and tumor agnostic studies and have been shown to predict benefit from various ICI ( 9 , 13 , 30 , 31 ). More recently, the FDA has approved pembrolizumab for patients with any tumor type that demonstrates a high TMB (≥10 mut/Mb) following results of the KEYNOTE-158 trial ( 34 ). Studies evaluating the combination of PD-L1 and TMB as a composite biomarker have suggested improved predictive capability for the combination ( 31 , 35 ).
There are no clinically useful biomarkers to help guide the use of ICIs in extensive stage SCLC. Despite presenting with immune-mediated paraneoplastic syndromes and often exhibiting high TMB, SCLC does not typically demonstrate the same clinical benefit to checkpoint inhibition that is seen in NSCLC. The landmark clinical trials establishing the role of ICI in combination with chemotherapy in first-line treatment of extensive-stage SCLC did not demonstrate any discernible difference in response rates or clinical utility of ICI for any PD-L1 subgroup ( 36 , 37 ).
Specific Genomic Alterations
There is some data to suggest that specific genomic alterations may predict better or worse responses to ICI. Much of this research has focused on determining genomic markers for immunotherapy resistance. STK11/LKB1 is a distinct subgroup of Kirsten Rat Sarcoma Virus (KRAS)- mutant lung adenocarcinoma. Some studies have suggested that the presence of KRAS mutation is a predictive of superior response rates from ICIs ( 38 ). However, specific co-mutations such as STK11/LKB1 have demonstrated poor immunogenic responses. In two separate lung adenocarcinoma cohorts, patients with KRAS and STK11/LKB1 co-mutations showed lower ORR compared with KRAS mutation alone and one of the cohorts noted a significantly lower PFS and OS during immunotherapy treatment ( 39 ). A second retrospective trial of patients with STK11 alone and STK11/KRAS co-mutations treated with immunotherapy showed poorer OS and PFS for those with STK11 mutations versus their STK11-wildtype counterparts ( 40 ). However, this study also notes poorer outcomes for STK11-mutant patients when treated with chemotherapy, and an additional analysis suggests that poor outcomes in this population are prognostic rather than predictive of poor immune response ( 41 )
Data has also been presented for ICI response in patients with common ‘targetable’ genomic alterations such as EGFR mutations and ALK fusions. Retrospective studies have demonstrated very low response rates with ICI in EGFR mutant NSCLC compared to EGFR wildtype tumors ( 42 , 43 ). Prospective trials of ICI monotherapy in patients with EGFR mutant NSCLC have yielded similarly disappointing results ( 36 , 44 ). Though the data is more scarce, patients with ALK gene rearrangements also appear to demonstrate poor responses to ICI monotherapy ( 36 , 45 ).
Circulating Tumor DNA (ctDNA)
Analysis of any tumor-derived material circulating in the peripheral blood, also called ‘Liquid Biopsy’, is gaining increasing popularity in oncology because of its feasibility and relatively rapid turnaround time. Of the multitude of blood-based biomarker analyses, circulating tumor DNA (ctDNA) is the most commonly used modality ( 46 ). The role of ctDNA analysis in the context of ICI therapy in lung cancer is currently limited to detection of specific genomic alterations noted above. Several commercial platforms are offering blood-based TMB assays, however, their clinical utility is yet to be validated in large prospective trials. Early data from studies looking at serial monitoring of ctDNA as a biomarker of response and survival with ICI show promising correlation between molecular and radiographic response ( 47 ). With well-designed future studies on a larger scale, it is conceivable that the depth of molecular response will guide the optimum duration of ICI therapy in future, a question that remains largely unanswered at this time.
Challenges Facing ICI Therapy in Lung Cancer
The unprecedented success of ICIs in the treatment of lung cancer is not without several major challenges. Firstly, nearly 70% of patients with advanced NSCLC and 80% of patients with SCLC do not derive durable benefit from ICI based therapies. Putative mechanisms of inherent or acquired resistance to ICI include T-cell exhaustion, co-expression of inhibitory receptors, altered metabolism through Indoleamine 2, 3-dioxygenase 1 (IDO-1) and increased adenosine production, high copy number loss of tumor suppressor genes, and decreased antigen presentation ( 48 ). Second, as noted above, the quest for a perfect and reliable biomarker of response to ICI is still ongoing. While PD-L1 expression is the most widely used biomarker in clinical practice, its predictive value is not absolute. Inter-tumor and intra-tumor heterogeneity of PD-L1 expression, a wide variety of PD-L1 assays and cutoff values utilized to define PD-L1 positivity, and the effect of handling and storage of tumor tissue on PD-L1 analysis render it an imperfect biomarker ( 11 , 49 – 52 ). Studies have shown significant discordance in PD-L1 expression between lung biopsies and corresponding resected tumors, between primary and metastatic sites, and among different metastatic sites ( 53 – 55 ). Finally, SCLC remains an invincible enemy with only a limited success achieved with incorporation of currently available ICIs in the treatment paradigm, largely driven by an immunosuppressive tumor microenvironment (TME) ( 56 ). These challenges have warranted development of novel approaches to harness the power of the immune system in combating this historically relentless disease.
Immunotherapy Approaches Beyond PD-1/PD-L1 and CTLA-4 Inhibition in Lung Cancer
Novel immune checkpoint targets.
As noted above, PD-1/PD-L1 and CTLA-4 inhibitors are the most commonly used ICIs in lung cancer, yet development of resistance to these agents remains an insurmountable challenge. In recent years, inhibitors of novel immune checkpoint targets have shown encouraging results in pre-clinical and early clinical studies, potentiating the pursuit of new therapeutic strategies to overcome the resistance to conventional ICIs.
T-Cell Immunoreceptor With Ig and ITIM Domains (TIGIT)
TIGIT is a promising new immune checkpoint. It is expressed on activated T cells, natural killer (NK) cells, and regulatory T cells (Tregs). TIGIT binds to two ligands, CD155 (PVR) and CD112 (PVRL2, nectin-2), that are expressed by tumor cells and antigen-presenting cells in the tumor microenvironment ( 57 ). Dual PD-1/TIGIT blockade potently increases tumor antigen-specific CD8+ T cell expansion and function in vitro and promotes tumor rejection in mouse tumor models ( 58 , 59 ). In a recently reported randomized phase II trial, combination of anti-TIGIT antibody tiragolumab with atezolizumab led to clinically meaningful improvement in ORR and PFS compared to placebo plus atezolizumab as first-line treatment of patients with advanced, PD-L1 positive NSCLC ( 60 ). Based on these results FDA has granted breakthrough therapy designation to tiragolumab in NSCLC. A confirmatory phase III trial is ongoing in this patient population (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT04294810","term_id":"NCT04294810"}} NCT04294810 ). Early results from a phase I trial of another anti-TIGIT antibody vibostolimab has shown clinical activity in combination with pembrolizumab in PD-1/PD-L1 naïve and refractory patients with advanced NSCLC ( 61 ). An ongoing phase III trial is comparing combination of vibostolimab plus pembrolizumab with pembrolizumab alone in patients with PD-L1 positive advanced NSCLC (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT04738487","term_id":"NCT04738487"}} NCT04738487 ).
Lymphocyte Activation Gene-3 (LAG-3)
LAG-3 is expressed on activated CD4+ and CD8+ T cells, Tregs, a subpopulation of NK cells, B cells, and plasmacytoid dendritic cells (pDCs). LAG-3 signaling plays a negative regulatory role in T helper 1 (Th1) cell activation, proliferation and cytokine secretion, a function that is exploited by tumor cells to evade the host immune system ( 62 ). Among several different LAG-3 inhibitors under development, monoclonal antibody relatlimab, is farthest along in clinical trials. The combination of relatlimab with nivolumab has shown significant improvement in PFS compared to nivolumab alone in patients with melanoma ( 63 ). A phase II trial evaluating the efficacy of relatlimab in combination with nivolumab and chemotherapy as first-line treatment of advanced NSCLC is currently ongoing (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT04623775","term_id":"NCT04623775"}} NCT04623775 ).
T-Cell Immunoglobulin and Mucin-Domain Containing-3 (TIM-3)
TIM-3, a negative regulator of T cell response and also called hepatitis A virus cellular receptor 2 (HAVCR2), expressed on CD4+ and CD8+ T cells, NK cells, DCs, Tregs, monocytes, and macrophages, is another emerging immune checkpoint ( 64 ). Higher expression of TIM-3 has been associated with poor prognosis in solid malignancies and inhibition of TIM-3 in combination with PD-1 inhibition has been shown to have anti-tumor activity ( 65 , 66 ). At least eight different TIM-3 inhibitors are in development and have shown superior efficacy of simultaneous inhibition of TIM-3 pathway and PD-1 pathway over single-agent treatment ( 67 ).
NK Group 2 Member A (NKG2A)
NKG2A is a cell surface molecule, which is typically expressed by NK cells, but expression can be induced on T cells as well, especially on CD8+ T cells ( 68 ). HLA-E, a ligand of NKG2A, expression has been demonstrated to have immunosuppressive function through binding to NKG2A ( 69 ). Overexpression of HLA-E on cancer cells has been correlated with poor outcomes ( 70 ). Monalizumab, a monoclonal antibody targeting NKG2A, has shown promising anti-tumor activity in early clinical trials in lung cancer, including recently reported interim analysis of a phase II trial showing improved ORR and PFS with monalizumab in combination with durvalumab compared to durvalumab alone in patients with unresectable, Stage III NSCLC who did not progress after concurrent chemoradiation therapy ( 71 ).
CD73, an ecto-5′-nucleotidase (NT5E), serves as an immune checkpoint by generating adenosine which suppresses immune activation through the A2A receptor ( 72 ). CD73 is upregulated in a variety of tumors including lung cancer, and higher expression of CD73 in the tumor tissue is associated with poor outcomes ( 72 – 75 ). Preclinical studies have established a strong foundation for evaluating CD73 inhibition in combination with PD-1/PD-L1 inhibition by demonstrating synergistic anti-tumor activity through augmentation of intra-tumoral infiltration of CD8+ tumor-specific T cells ( 76 , 77 ). Recently reported early results of a phase II clinical trial evaluating oleclumab, a monoclonal antibody against CD73, in combination with durvalumab following chemoradiation in locally advanced unresectable stage III NSCLC showed improved PFS with the combination compared to durvalumab alone, with a manageable safety profile ( 78 ). A number of trials are underway to evaluate oleclumab containing regimens in lung cancer.
Apart from these, several other immune checkpoint targets are under investigation, including V-domain immunoglobulin suppressor of T cell activation (VISTA), B7-H3 (CD276), IDO-1, glucocorticoid-induced TNFR-related receptor (GITR), and CD47. A multitude of ongoing clinical trials are evaluating inhibitors of these targets by themselves and in combination with PD-1/PD-L1 inhibition ( Table 1 ).
Table 1
Novel immune checkpoint inhibitors and ongoing clinical trials.
TIGIT, T cell immunoreceptor with Ig and ITIM domains; mAb, monoclonal antibody; LAG-3, Lymphocyte activation gene 3; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; NKG2A, NK group 2 member A; CD73, Cluster of differentiation 73; IDO1, Indoleamine 2, 3-dioxygenase 1; B7-H3, B7 homolog 3; IFN- γ, Interferon Gamma; CD27, Cluster of differentiation 27; GITR, glucocorticoid-induced TNFR-related receptor; CD47 and SIRPα, Cluster of differentiation 47 and Signal Regulatory Protein Alpha; VISTA, V-domain immunoglobulin suppressor of T cell activation; IL-2, Interleukin 2; T-regs, Regulatory T-cells; NK cells, Natural Killer Cells; PD-L1, Programmed Death-Ligand 1.
Combination with Conventional Therapeutic Strategies
Chemotherapy.
As noted above, chemotherapy in combination with ICI has shown superiority over chemotherapy alone in several randomized controlled trials in NSCLC and SCLC. In these trials, patients typically received 4 cycles of platinum-based chemotherapy in combination with ICI followed by maintenance ICI alone or pemetrexed plus ICI in case of non-squamous NSCLC. Subsequently, CheckMate-9LA trial demonstrated feasibility of using shorter duration, i.e. 2 cycles, of chemotherapy in combination with PD-1 and CTLA-4 inhibitors without comprising the efficacy of the regimen ( 17 ). While the synergistic activity of ICIs in combination with chemotherapy is well established and that the addition of ICI may allow for shorter duration of chemotherapy, several questions remain unanswered, including the optimum number of chemotherapy cycles in the initial induction phase, whether pemetrexed continuation is critical in the maintenance phase for non-squamous NSCLC, predictive biomarkers to inform decision making with regards to the duration of chemoimmunotherapy, and value of adding ICI to second-line chemotherapy following progression on first-line chemoimmunotherapy.
The immunomodulatory effects of radiation are well established and include a shift in tumor associated macrophage polarization, activation of tumor-associated dendritic cells, improved T-cell homing to tumors, destruction of immunosuppressive stromal cells within the TME, and induction of immunogenic cell death ( 79 ). Consequently, combining ICI with radiation, either concurrently or sequentially, has been an area of great interest. A number of clinical trials have been initiated to build on the success of PACIFIC trial with consolidation durvalumab following chemoradiation in patients with locally advanced unresectable NSCLC. A notable concern with combining ICI with radiation is the development of toxicity, specifically pneumonitis. In a phase II trial of pembrolizumab with concurrent chemoradiation, the rate of grade ≥ 3 pneumonitis was 8% compared to 3.4% reported in PACIFIC trial ( 80 ). Future approaches directed towards modifying the dosing and schedule of radiation when used in close proximity with ICI may enhance the feasibility and minimize toxicity of this approach.
Targeted Therapy
The list of actionable genomic alterations in NSCLC and corresponding targeted therapeutic approaches have expanded considerably in the recent years. Retrospective studies and subset analyses of prospective trials have shown limited efficacy of ICIs in patients with NSCLC harboring targetable driver alterations ( 43 , 81 ). Combination of ICIs with EGFR and ALK tyrosine kinase inhibitors has shown increase in severe treatment related toxicity, including pneumonitis and liver dysfunction, with no added clinical activity ( 82 ). KRAS mutation is perhaps an exception to this limitation. KRAS G12C inhibitors have shown development of pro-inflammatory TME and durable responses alone as well as in combination with ICIs in mice models ( 83 ). Clinical trials combining ICIs with currently available KRAS G12C inhibitors, sotorasib and adagrasib, are ongoing (ClinicalTrials.gov identifiers: {"type":"clinical-trial","attrs":{"text":"NCT04185883","term_id":"NCT04185883"}} NCT04185883 , {"type":"clinical-trial","attrs":{"text":"NCT04613596","term_id":"NCT04613596"}} NCT04613596 ).
Combination With DNA Repair Targeting Agents
DNA damage is a hallmark of lung cancer and is most apparent in smoking induced NSCLC and SCLC ( 84 ). Smoking induced DNA damage triggers several DNA response pathways ( 85 ). Accurate and faithful DNA replication is critical for maintenance of genomic stability in all cellular divisions including that of immune cells ( 86 , 87 ). While traditionally DNA repair has been studied in the context of sensitivity to platinum-based chemotherapy and PARP inhibition, mounting evidence suggests the role of DNA repair defects in predicting response to ICIs in a variety of tumors, including NSCLC ( 88 – 91 ). A well-known example is the efficacy of pembrolizumab in mismatch repair deficient tumors irrespective of PD-L1 expression ( 92 ). Germline mutations in BRCA2 or POLE have also been connected to increased ICI sensitivity ( 93 , 94 ). A retrospective study has shown strong association of somatic BRCA1 , PALB2 , and POLE mutations with high TMB in NSCLC ( 95 ). The precise mechanisms behind the association of DNA repair defects and response to ICI, however, remain poorly understood. Genomic instability resulting from these defects, higher neoantigen load and TMB, and association with activation of cGAS-STING pathway are among the postulated key players ( 90 , 96 ).
In addition to utilizing DNA repair defects in predicting response to ICI in lung cancer, an increasing amount of research is focusing on the prospect of combining ICIs with DNA repair targeting agents in NSCLC. Perhaps the farthest along in this race are PARP inhibitors and ATR inhibitors. Preclinical studies have shown upregulation of PD-L1 expression with PARP inhibition, re-sensitization of PARP inhibitor treated cancer cells to T-cell mediated killing through PD-L1 blockade, and enhanced in vivo therapeutic efficacy of PARP inhibition in combination with anti-PD-L1 therapy ( 97 ). Three large ongoing clinical trials are evaluating the efficacy and safety of combination of ICI with PARP inhibitor in NSCLC, including KEYLINK-006 (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03976323","term_id":"NCT03976323"}} NCT03976323 ), KEYLINK-008 (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03976362","term_id":"NCT03976362"}} NCT03976362 ), and ORION (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03775486","term_id":"NCT03775486"}} NCT03775486 ). Similarly, a trial of ATR inhibitor ceralasertib evaluating its combination with paclitaxel in patients with a variety of solid organ malignancies showed upregulation of PD-L1 expression in paired tumor samples following treatment with ceralasertib ( 98 ). As a result of this initial finding, an ongoing study is evaluating combination of ceralasertib with durvalumab in patients with advanced NSCLC following progression on ICI therapy (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT03334617","term_id":"NCT03334617"}} NCT03334617 ). While these studies are being conducted in all NSCLC patients, it remains unclear whether the benefit of such combinations will be restricted to those with DNA repair defective tumors. Additionally, clinically feasible and reproducible biomarkers of DNA repair defects in lung cancer will need to be evaluated and validated in prospective studies.
Figure 1 illustrates novel immunotherapy targets and intersection with DNA repair in lung cancer.

Novel immunotherapy targets and intersection with DNA repair.
Cellular Therapy in Lung Cancer
Immune cell-based therapy has recently emerged as a promising immunotherapeutic approach to target lung cancer ( 99 ). Building on successes in other tumors, immune cells have been exploited for their innate ability to eliminate cancer cells and mount powerful immune responses by recruiting other cells in the TME. To enhance specificity of immune recognition of cancer cells, genetic engineering of T- or NK cells has enabled these cells to target specific antigens expressed on lung cancer cells and reprogram the immune cells’ behavior toward enhanced function. Most of the studies on immune cell-based targeting of lung cancer have been reported for CAR-T cells, though a growing body of work is exploiting the allogeneic nature of NK cells as potentially safer alternatives to infused CAR-T cells ( 100 ).
Engineered cell-based therapies for lung cancer have so far been designed to target epidermal growth factor receptor (EGFR) and variant III (EGFRviii), glypican 3 (GPC3), human epidermal growth factor receptor 2 (HER2), Protein tyrosine kinase 7 (PTK7), erythropoietin-producing hepatocellular carcinoma A2 (EphA2), mesothelin (MSLN), prostate stem cell antigen (PSCA), mucin 1 (MUC1), carcinoembryonic antigen (CEA), natural killer group 2D (NKG2D), tyrosine kinase-like orphan receptor 1 (ROR1), and programmed cell death ligand 1 (PD-L1),lung-specific X (LunX), and delta-like 3 (DLL3) ( 101 – 104 ).
Future clinical development of cellular therapies targeting many of these antigens has been challenged by toxicities observed upon infusion of CAR-T products. For instance, infusion of ERBB2-specific CAR-T cells to treat a patient with colon cancer with metastasis to lungs and liver caused respiratory distress within 15 minutes of cell infusion ( 105 ). The authors of the study speculated that this was due to the high activity of CAR-T cells in lungs following recognition of low levels of ERBB2 expression on lung epithelial cells, thus triggering intense, and toxic, cytokine production. Acute respiratory toxicities were also observed in a cohort of patients treated with CEACAM-5-directed CAR-T cells, again due to expression of CEACAM-5 on the lung epithelium ( 106 ). This was accompanied by high levels of IFN-γ and IL-6. This lack of specificity for tumor expressed antigens is one of the major challenges to the clinical development of CAR-T cell therapies in solid tumors, specifically lung cancer.
Though the ideal antigen is one that is exclusively present on tumor cells and absent in healthy tissue, targeting approaches often must grapple with sub-optimal expression of targetable antigens due to their presence in surrounding healthy tissues. In addition to the non-specific nature of expression of many of the targetable antigens in lung cancer, antigen density is highly heterogeneous in the tumors, and their high genomic instability leads to antigen loss or outgrowth variants that can occur in response to persistence targeting by antigen-specific CAR-T cells ( 107 ). Antigen loss has spurred the development of engineering strategies that can induce genetic circuitry to immune cells and avoid antigen escape. These include dual or multispecific CAR-T cells, CAR-T cell secreting antibodies such as PD-L1, as well as trigger-responsive CAR-T cells (e.g. synNotch CAR-T cells, which are designed to target multiple antigens and enhance their specificity against the tumor ( 108 , 109 ).
Despite the demonstrated successes with cell-based therapies with hematological malignancies, the response rates in solid tumors, including lung cancer, have been underwhelming. In lung cancer, the TME presents a complex barrier to the activity of immune cells which often results in resistance to treatment. The homing of CAR-T cells is poor due to several factors acting against them in the lung cancer TME. These include inadequate or mismatched chemokine-chemokine receptor pairs, downregulation of adhesion molecules, aberrant vasculature, unfavorable extracellular matrix (ECM) composition, and immunometabolically adverse conditions such as hypoxia and the presence of immunosuppressive soluble metabolites and factors such as TGF-β, lactate and adenosine. In particular, in lung cancer, the structure of the ECM, composed of collagens, proteoglycans and glycosaminoglycans, has been reported as playing a significant role in the ability of immune cells to successfully home to lung tumors ( 110 ). In addition, dysfunction of infiltrating immune cells occurs due to unfavorable immunometabolic conditions in the tumor. Dysfunction, in the case of CAR-T cells, can manifest as exhaustion, senescence, or anergy ( 111 ). This results in inadequate immune responses and has triggered the development of “exhaustion-resistant” CAR-T cells, which are engineered by knocking out genes that contribute to T cell dysfunction, such as, in one case, TCR, HLA class I, PD-1, and CTLA-4 using one-shot CRISPR ( 112 ). Recent studies have also shown that blocking the adenosine pathway by inhibiting activity of CD73 can enhance immune cell activity and infiltration into lung cancer in preclinical mouse models ( 113 ). In these studies, NK cells engineered to target NKG2D on lung cancer were combined with antibody-mediated blockade of CD73 and resulted in deeper intratumoral infiltration and killing ability of these cells.
Finally, CAR-T cells themselves can cause toxicities upon systemic administration. Severe and sometimes lethal cytokine levels have been measured in patients treated with CAR T cells in many clinical trials, not exclusive to lung cancer ( 114 ). Mitigation measures include antibody therapy to reduce the burden of cytokine release syndrome, engineering safety switch-based CAR-T cells, or using NK cells. Local administration of immune cell therapies is currently under preclinical and clinical investigation as an approach to directly drive cells to the tumor.
The landscape of immunotherapy in lung cancer is rapidly expanding and ICIs have become the standard of care treatment for patients with metastatic, locally advanced, and resectable NSCLC with remarkable improvement in OS. The clinical utility of ICI in SCLC is limited to first-line therapy of extensive-stage disease with small improvement in OS. Resistance to immunotherapy, either inherent or acquired, is a major challenge facing the Oncology community. Cellular therapy is a promising and potent addition to the arsenal of immunotherapies for lung cancer. Lack of tumor-specific antigens, hostile TME, and toxicity make cellular therapy an exciting, but undeniably challenging, proposition. Development of novel treatment strategies, including combination and sequencing of PD-1/PD-L1 inhibitors with other ICIs and DNA repair targeting agents, are being evaluated in clinical trials.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors have following conflicts of interest within the past 2 years, none of which influenced the work being submitted. HM: Advisory role – Zentalis. GD: Research grant - BMS, AstraZeneca, Merck; Honoraria - AstraZeneca, Curio Science. SJ: Research grant - AstraZeneca, Astex, Tesaro; Consultant – Adaptimmune.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Advances in Lung Cancer Research
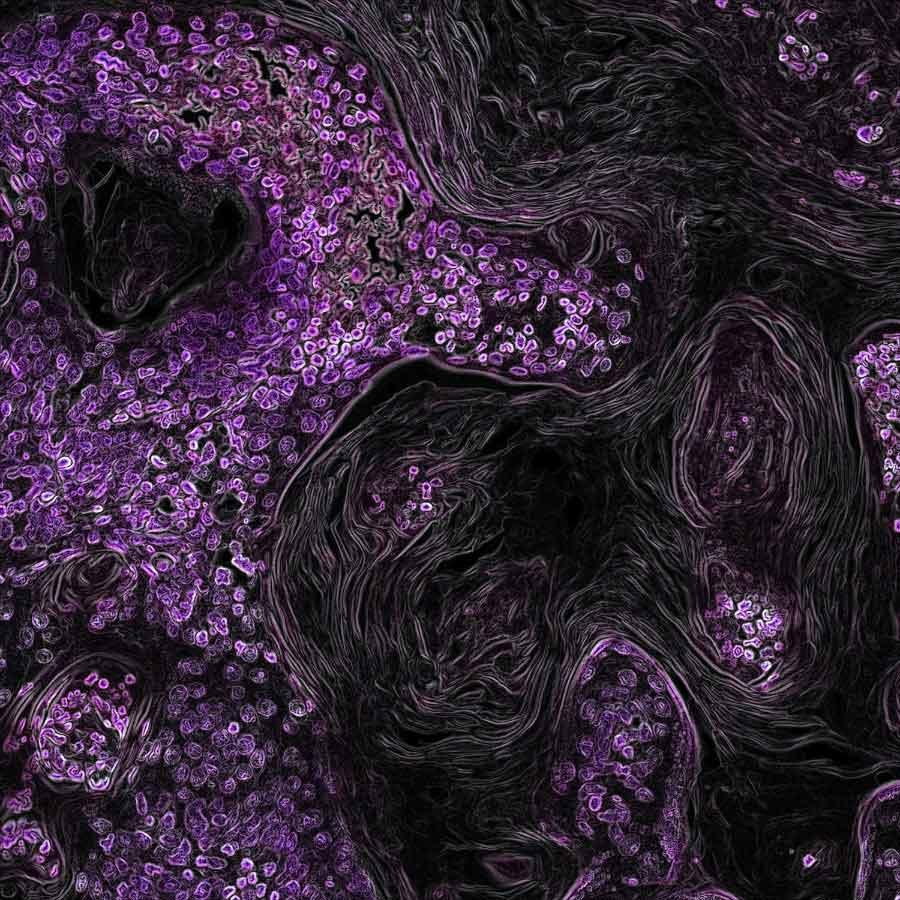
Lung cancer cells driven by the KRAS oncogene, which is highlighted in purple.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat lung cancer. In particular, scientists have made progress in identifying many different genetic alterations that can drive lung cancer growth.
This page highlights some of the latest research in non-small cell lung cancer (NSCLC), the most common form of lung cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
Early Detection of Lung Cancer
A great deal of research has been conducted in ways to find lung cancer early. Several methods are currently being studied to see if they decrease the risk of dying from lung cancer.
The NCI-sponsored National Lung Screening Trial (NLST) showed that low-dose CT scans can be used to screen for lung cancer in people with a history of heavy smoking. Using this screening can decrease their risk of dying from lung cancer. Now researchers are looking for ways to refine CT screening to better predict whether cancer is present.
Markers in Blood and Sputum
Scientists are trying to develop or refine tests of sputum and blood that could be used to detect lung cancer early. Two active areas of research are:
- Analyzing blood samples to learn whether finding tumor cells or molecular markers in the blood will help diagnose lung cancer early.
- Examining sputum samples for the presence of abnormal cells or molecular markers that identify individuals who may need more follow-up.
Machine Learning
Machine learning is a method that allows computers to learn how to predict certain outcomes. In lung cancer, researchers are using computer algorithms to create computer-aided programs that are better able to identify cancer in CT scans than radiologists or pathologists. For example, in one artificial intelligence study , researchers trained a computer program to diagnose two types of lung cancer with 97% accuracy, as well as detect cancer-related genetic mutations.
Lung Cancer Treatment
Treatment options for lung cancer are surgery , radiation , chemotherapy , targeted therapy , immunotherapy , and combinations of these approaches. Researchers continue to look for new treatment options for all stages of lung cancer.

Treatments for early-stage lung cancer
Early-stage lung cancer can often be treated with surgery. Researchers are developing approaches to make surgery safer and more effective.
- When lung cancer is found early, people usually have surgery to remove an entire section ( lobe ) of the lung that contains the tumor. However, a recent clinical trial showed that, for certain people with early-stage NSCLC, removing a piece of the affected lobe is as effective as surgery to remove the whole lobe .
- The targeted therapy Osimertinib (Tagrisso ) was approved by the Food and Drug Administration (FDA) in 2021 to be given after surgery—that is, as adjuvant therapy —to people with early-stage NSCLC that has certain mutations in the EGFR gene.
- Two immunotherapy drugs, atezolizumab (Tecentriq) and pembrolizumab (Keytruda) have been approved by the FDA to be used as adjuvant treatments after surgery and chemotherapy, for some patients with early-stage NSCLC.
- The immunotherapy drug nivolumab (Opdivo) is approved to be used, together with chemotherapy, to treat patients with early-stage lung cancer before surgery (called neoadjuvant ). This approval, which came in 2022, was based on the results of the CheckMate 816 trial, which showed that patients at this stage who received neoadjuvant nivolumab plus chemotherapy lived longer than those who received chemotherapy alone .
- In another trial (Keynote-671), patients with early-stage NSCLC who received pembrolizumab plus chemotherapy before surgery and pembrolizumab after surgery had better outcomes than those who received just neoadjuvant or just adjuvant treatment.
Treatments for advanced lung cancer
Newer therapies are available for people with advanced lung cancer. These primarily include immunotherapies and targeted therapies, which continue to show benefits as research evolves.
Immunotherapy
Immunotherapies work with the body's immune system to help fight cancer. They are a major focus in lung cancer treatment research today. Clinical trials are ongoing to look at new combinations of immunotherapies with or without chemotherapy to treat lung cancer.
Immune checkpoint inhibitor s are drugs that block an interaction between proteins on immune cells and cancer cells which, in turn, lowers the immune response to the cancer. Several immune checkpoint inhibitors have been approved for advanced lung cancer, including p embrolizumab (Keytruda) , a tezolizumab (Tecentriq) , c emiplimab (Libtayo) , d urvalumab (Imfinzi) , and n ivolumab (Opdivo) .
A key issue with immunotherapies is deciding which patients are most likely to benefit. There is some evidence that patients whose tumor cells have high levels of an immune checkpoint protein called PD-L1 may be more responsive to immune checkpoint inhibitors. Another marker for immunotherapy response is tumor mutational burden , or TMB, which refers to the amount of mutations in the DNA of the cancer cells. In some lung cancer trials, positive responses to immune checkpoint inhibitors have been linked with a high TMB. However, these markers cannot always predict a response and there is ongoing work to find better markers.
To learn more, see Immunotherapy to Treat Cancer .
Targeted Therapies
Targeted treatments identify and attack certain types of cancer cells with less harm to normal cells. In recent years, many targeted therapies have become available for advanced lung cancer and more are in development. Targeted treatments for lung cancer include the below.
Anaplastic lymphoma kinase (ALK) Inhibitors
ALK inhibitors target cancer-causing rearrangements in a protein called ALK. These drugs continue to be refined for the 5% of NSCLC patients who have an ALK gene alteration. Approved treatments include ceritinib (Zykadia) , alectinib (Alecensa) , brigatinib (Alunbrig) , and lorlatinib (Lorbrena) .
These ALK inhibitors are improvements from previous ones in their enhanced ability to cross the blood–brain barrier. This progress is critical because, in non-small cell lung cancer patients with ALK alterations, disease progression tends to occur in the brain. Based on clinical trial results, in 2024 the FDA approved alectinib as adjuvant therapy for people with ALK-positive NSCLC .
EGFR Inhibitors
- Lung Cancer Trial of Osimertinib Draws Praise—and Some Criticism
The drug improved survival in a large clinical trial, but some question the trial’s design.
EGFR inhibitors block the activity of a protein called epidermal growth factor receptor (EGFR). Altered forms of EGFR are found at high levels in some lung cancers, causing them to grow rapidly. Osimertinib (Tagrisso) is the most effective and most widely used EGFR inhibitor. It is also used for adjuvant therapy after surgery for resectable NSCLC. Other drugs that target EGFR that are approved for treating NSCLC include afatinib (Gilotrif) , dacomitinib (Vizimpro) , erlotinib (Tarceva) , gefitinib (Iressa) . For people with Exon 20 mutations, amivantamab (Rybrevant) is an approved targeted therapy.
ROS1 Inhibitors
The ROS1 protein is involved in cell signaling and cell growth. A small percentage of people with NSCLC have rearranged forms of the ROS1 gene. Crizotinib (Xalkori) and entrectinib (Rozlytrek) are approved as treatments for patients with these alterations. In late 2023, the FDA approved repotrectinib (Augtyro) for advanced or metastatic NSCLC with ROS1 fusions as an initial treatment and as a second-line treatment in those who previously received a ROS1-targeted drug.
BRAF Inhibitors
The B-Raf protein is involved in sending signals in cells and cell growth. Certain changes in the B-Raf gene can increase the growth and spread of NSCLC cells.
The combination of the B-Raf-targeted drug dabrafenib (Tafinlar) and trametinib (Mekinist ), which targets a protein called MEK, has been approved as treatment for patients with NSCLC that has a specific mutation in the BRAF gene.
Encorafenib (Braftovi) combined with binimetinib (Mektovi) is approved for patients with metastatic NSCLC with a BRAF V600E mutation .
Other Inhibitors
Some NSCLCs have mutations in the genes NRTK-1 and NRTK-2 that can be treated with the targeted therapy larotrectinib (Vitrakvi). Those with certain mutations in the MET gene can be treated with tepotinib (Tepmetko) or capmatinib (Tabrecta) . And those with alterations in the RET gene are treated with selpercatinib (Retevmo) and pralsetinib (Gavreto) . A 2023 clinical trial showed that treatment with selpercatinib led to longer progression-free survival compared with people who received chemotherapy with or without pembrolizumab. Inhibitors of other targets that drive some lung cancers are being tested in clinical trials.
See a complete list of targeted therapies for lung cancer .
NCI-Supported Research Programs
Many NCI-funded researchers at the NIH campus, and across the United States and the world, are seeking ways to address lung cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate basic information into improved patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in lung cancer.
Pragmatica-Lung Study Enrolling Patients
The simplified trial may serve as a model for future cancer clinical trials.
- The Pragmatica-Lung Study is a randomized trial that will compare the combination of the targeted therapy ramucirumab (Cyramza) and the immunotherapy pembrolizumab (Keytruda) with standard chemotherapy in people with advanced NSCLC whose disease has progressed after previous treatment with immunotherapy and chemotherapy. In addition to looking at an important clinical question, the trial will serve as a model for future trials because it is designed to remove many of the barriers that prevent people from joining clinical trials.
- Begun in 2014, ALCHEMIST is a multicenter NCI trial for patients with early stage non-small cell lung cancer. It tests to see whether adding a targeted therapy after surgery, based on the genetics of a patient’s tumor, will improve survival.
- The Lung MAP trial is an ongoing multicenter trial for patients with advanced non-small cell lung cancer who have not responded to earlier treatment. Patients are assigned to specific targeted therapies based on their tumor’s genetic makeup.
- The Small Cell Lung Cancer Consortium was created to coordinate efforts and provide a network for investigators who focus on preclinical studies of small-cell lung cancer. The goal of the consortium is to accelerate progress on this disease through information exchange, data sharing and analysis, and face-to-face meetings.
- NCI funds eight lung cancer Specialized Programs of Research Excellence (Lung SPOREs) . These programs are designed to quickly move basic scientific findings into clinical settings. Each SPORE has multiple lung cancer projects underway.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for both non-small cell lung cancer treatment and small cell lung cancer treatment .
Lung Cancer Research Results
The following are some of our latest news articles on lung cancer research:
- Alectinib Approved as an Adjuvant Treatment for Lung Cancer
- Repotrectinib Expands Treatment Options for Lung Cancers with ROS1 Fusions
- Tarlatamab Shows Promise for Some People with Small Cell Lung Cancer
- Selpercatinib Slows Progression of RET-Positive Lung, Medullary Thyroid Cancers
- Lung-Sparing Surgery Is Effective for Some with Early-Stage Lung Cancer
View the full list of Lung Cancer Research Results and Study Updates .
- My Shodhganga
- Receive email updates
- Edit Profile
Shodhganga : a reservoir of Indian theses @ INFLIBNET
- Shodhganga@INFLIBNET
- Anna University
- Faculty of Information and Communication Engineering
Items in Shodhganga are licensed under Creative Commons Licence Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0).

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News & Views
- Published: 13 May 2024
Cancer therapy
Refining neoadjuvant immunotherapy for resectable lung cancer
- Misty D. Shields 1 &
- Christine M. Lovly ORCID: orcid.org/0000-0002-3641-6361 2
Nature Medicine ( 2024 ) Cite this article
68 Accesses
14 Altmetric
Metrics details
- Drug development
In an era of expanding perioperative approaches for resectable non–small-cell lung cancer, new data demonstrate that dual neoadjuvant immunotherapy targeting PD-1 and LAG-3 is feasible; future analyses may enhance patient selection by identifying immune signatures predictive of response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Sung, H. et al. CA Cancer J. Clin. 71 , 209–249 (2021).
Article PubMed Google Scholar
The National Lung Screening Trial Research Team. N. Engl. J. Med. 365 , 395–409 (2011).
Article PubMed Central Google Scholar
Pignon, J. P. et al. J. Clin. Oncol. 26 , 3552–3559 (2008).
Desai, A. P., Adashek, J. J., Reuss, J. E., West, H. J. & Mansfield, A. S. JAMA Oncol. 9 , 135–142 (2023).
Sorin, M. et al. JAMA Oncol . https://doi.org/10.1001/jamaoncol.2024.0057 (2024).
Schuler, M. Nat. Med. https://doi.org/10.1038/s41591-024-02965-0 (2024).
Wakelee, H. et al. N. Engl. J. Med. 389 , 491–503 (2023).
Article CAS PubMed Google Scholar
Forde, P. M. et al. N. Engl. J. Med. 386 , 1973–1985 (2022).
Article CAS PubMed PubMed Central Google Scholar
Pellini, B. & Chaudhuri, A. A. J. Clin. Oncol. 40 , 567–575 (2022).
Mino-Kenudson, M. et al. J. Thorac. Oncol. 17 , 1335–1354 (2022).
Skoulidis, F. et al. Cancer Discov. 8 , 822–835 (2018).
Qiao, M. et al. J. Thorac. Oncol. 16 , 1267–1288 (2021).
Download references
Author information
Authors and affiliations.
Department of Medicine, Division of Hematology and Oncology, Indiana University School of Medicine, Indianapolis, IN, USA
Misty D. Shields
Department of Medicine, Division of Hematology and Oncology, Vanderbilt University Medical Center, Nashville, TN, USA
Christine M. Lovly
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christine M. Lovly .
Ethics declarations
Competing interests.
M.D.S. previously served on the speakers’ bureau for Jazz Pharmaceuticals and has served on advisory board for Jazz Pharmaceuticals and AstraZeneca. C.M.L. has served on advisory boards for Amgen, AnHeart, Arrivent, AstraZeneca, Blueprints Medicine, BMS, Boehringer Ingelheim, Cepheid, D2G, Daiichi Sankyo, EMD Serono, Foundation Medicine, Genentech, Gilead, Guardant, Indupro, Janssen, Medscape, Novartis, Pfizer, Regeneron, Roche, Takeda and Tempus and has also served on a data and safety monitoring board for Janssen (uncompensated).
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Shields, M.D., Lovly, C.M. Refining neoadjuvant immunotherapy for resectable lung cancer. Nat Med (2024). https://doi.org/10.1038/s41591-024-03001-x
Download citation
Published : 13 May 2024
DOI : https://doi.org/10.1038/s41591-024-03001-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Introduction
- Conclusions
- Article Information
The dotted lines indicate 10 years from time of cohort enrollment (A) and 10 years from time of IPLC diagnosis (B). Gray and orange shading indicates the 95% CI of cumulative incidence of IPLC (A) and SPLC (B).
The dotted lines indicate 10 years from time of cohort enrollment (A and C) and 10 years from time of IPLC diagnosis (B and D). Gray and orange shading indicates the 95% CI of cumulative incidence of IPLC (A and C) and SPLC (B and D).
eMethods. Interpretation of Standardized Incidence Ratio (SIR) vs Cause-Specific Cox Regression
eReferences
eTable 1. The Standardized Incidence Ratio in the Overall Cohort and by Smoking History
eTable 2. The Standardized Incidence Ratio in the Overall Cohort and by Smoking History, Stratified by Sex
eTable 3. The Standardized Incidence Ratio in the Overall Cohort and by Smoking History Based on Early-Stage Cases of Initial Primary Lung Cancer
eTable 4. The Standardized Incidence Ratio in the Overall Cohort and by Smoking History Based on Adenocarcinoma Initial Primary Lung Cancer
eTable 5. Sensitivity Analysis of the Adjusted Standardized Incidence Ratio in the Overall Cohort and by Smoking History Using Poisson Regression
eFigure 1. Cumulative Incidence of Early-Stage Initial Primary Lung Cancer in the General Population (n = 207 554), in A, and Cumulative Incidence of Second Primary Lung Cancer in Patients With Early-Stage Lung Cancer (n = 3289), in B
eFigure 2. Cumulative Incidence of Adenocarcinoma Initial Primary Lung Cancer in the General Population (n = 207 179), in A, and Cumulative Incidence of Second Primary Lung Cancer in Patients With Early-Stage Lung Cancer (n = 2874), in B
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Choi E , Su CC , Wu JT, et al. Second Primary Lung Cancer Among Lung Cancer Survivors Who Never Smoked. JAMA Netw Open. 2023;6(11):e2343278. doi:10.1001/jamanetworkopen.2023.43278
Manage citations:
© 2024
- Permissions
Second Primary Lung Cancer Among Lung Cancer Survivors Who Never Smoked
- 1 Quantitative Sciences Unit, Stanford University School of Medicine, Stanford, California
- 2 Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, California
- 3 Department of Medicine, Stanford University School of Medicine, Stanford, California
- 4 Department of Medicine, University of California, San Francisco
- 5 Stanford Cancer Institute, Stanford, California
- 6 Department of Radiology, Stanford University School of Medicine, Stanford, California
- 7 Department of Cardiothoracic Surgery, Stanford University School of Medicine, Stanford, California
- 8 Cancer Epidemiology Program, University of Hawaii Cancer Center, Honolulu
- 9 Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles
- 10 Sutter Health, Palo Alto Medical Foundation Research Institute, Palo Alto, California
- 11 Department of Epidemiology and Biostatistics, University of California, San Francisco
- 12 Department of Neurosurgery, Stanford University School of Medicine, Stanford, California
Question What is the risk of developing second primary lung cancer (SPLC) among lung cancer survivors who have never smoked?
Findings In this cohort study of 211 414 participants, the cumulative 10-year incidence of initial primary lung cancer (IPLC) among ever-smokers was 7 times higher than for never-smokers. However, the cumulative SPLC incidence following IPLC was as high among lung cancer survivors who never smoked as those who ever smoked.
Meaning These findings highlight the need to identify risk factors for SPLC among lung cancer survivors who never smoked and to develop a targeted surveillance strategy.
Importance Lung cancer among never-smokers accounts for 25% of all lung cancers in the US; recent therapeutic advances have improved survival among patients with initial primary lung cancer (IPLC), who are now at high risk of developing second primary lung cancer (SPLC). As smoking rates continue to decline in the US, it is critical to examine more closely the epidemiology of lung cancer among patients who never smoked, including their risk for SPLC.
Objective To estimate and compare the cumulative SPLC incidence among lung cancer survivors who have never smoked vs those who have ever smoked.
Design, Setting, and Participants This population-based prospective cohort study used data from the Multiethnic Cohort Study (MEC), which enrolled participants between April 18, 1993, and December 31, 1996, with follow-up through July 1, 2017. Eligible individuals for this study were aged 45 to 75 years and had complete smoking data at baseline. These participants were followed up for IPLC and further SPLC development through the Surveillance, Epidemiology, and End Results registry. The data were analyzed from July 1, 2022, to January 31, 2023.
Exposures Never-smoking vs ever-smoking exposure at MEC enrollment.
Main Outcomes and Measures The study had 2 primary outcomes: (1) 10-year cumulative incidence of IPLC in the entire study cohort and 10-year cumulative incidence of SPLC among patients with IPLC and (2) standardized incidence ratio (SIR) (calculated as the SPLC incidence divided by the IPLC incidence) by smoking history.
Results Among 211 414 MEC participants, 7161 (3.96%) developed IPLC over 4 038 007 person-years, and 163 (2.28%) developed SPLC over 16 470 person-years. Of the participants with IPLC, the mean (SD) age at cohort enrollment was 63.6 (7.7) years, 4031 (56.3%) were male, and 3131 (43.7%) were female. The 10-year cumulative IPLC incidence was 2.40% (95% CI, 2.31%-2.49%) among ever-smokers, which was 7 times higher than never-smokers (0.34%; 95% CI, 0.30%-0.37%). However, the 10-year cumulative SPLC incidence following IPLC was as high among never-smokers (2.84%; 95% CI, 1.50%-4.18%) as ever-smokers (2.72%; 95% CI, 2.24%-3.20%), which led to a substantially higher SIR for never-smokers (14.50; 95% CI, 8.73-22.65) vs ever-smokers (3.50; 95% CI, 2.95-4.12).
Conclusions and Relevance The findings indicate that SPLC risk among lung cancer survivors who never smoked is as high as among those with IPLC who ever-smoked, highlighting the need to identify risk factors for SPLC among patients who never smoked and to develop a targeted surveillance strategy.
Lung cancer remains the leading cause of cancer-related mortality in the US, causing more deaths than breast, prostate, and colon cancer combined. 1 While smoking is the predominant risk factor, approximately one-quarter of patients worldwide develop lung cancer without a smoking history. 2 - 4 Although the risk factors for lung cancer among never-smokers have not been thoroughly examined, prior studies have associated factors such as radon exposure, 5 secondhand smoke, 6 and genetic susceptability 7 with lung cancer in this patient population. Patients who have never smoked make up 10% to 15% of the lung cancer population in the US and Europe and up to 40% in Asia. 8 - 10 As smoking rates continue to decrease, 11 it is critical to closely examine the epidemiology of lung cancer among patients without a smoking history.
Concurrently, second primary lung cancer (SPLC) is an increasing public health concern. 12 The number of lung cancer survivors has been increasing with early detection through screening and therapeutic advances. However, lung cancer survivors have a 4- to 6-times higher risk of developing SPLC compared with the risk of developing initial primary lung cancer (IPLC) in the general population 13 ; the cumulative incidence of SPLC has increased continuously without plateau. 12 Although prior studies have examined SPLC risk factors, 14 - 16 such as surgical resection for IPLC 15 and tobacco smoking, 14 , 16 and have developed SPLC prediction models, 17 , 18 they lack insight into the patterns of SPLC incidence specifically among lung cancer survivors who never smoked. A few single-institution studies for SPLC have included never-smokers, but the numbers of cases were limited, 16 , 19 and, to our knowledge, no prior studies have characterized the patterns of SPLC incidence among never-smoking survivors using prospective, population-based cohort data. In this study, we aimed to characterize and quantify SPLC incidence and disease burden among lung cancer survivors who have never vs ever smoked from a large, population-based cohort with long-term prospective follow-up.
For this cohort study, data were derived from the Multiethnic Cohort Study (MEC), which includes a large, ethnically diverse cohort of 214 862 healthy adults in California and Hawaii. The MEC was established to examine key factors associated with cancer risk among adults aged 45 to 75 years at cohort enrollment (April 18, 1993, to December 31, 1996) across different racial groups with the following racial and ethnic distribution: African American (16.2%), Japanese American (26.4%), Latino (22.1%), Native Hawaiian (6.7%), White (23.0%), and other (5.7%). 20 At enrollment, participant characteristics were collected through a baseline self-report questionnaire that measured dietary and nondietary risk factors, including smoking, alcohol consumption, and physical activity. The receipt of a questionnaire was considered as consent to participate in the MEC by the institutional review boards of the University of Hawaii and the University of Southern California; all participating sites received a waiver of consent per institutional review board guidelines due to the absence of processes requiring written consent, the sharing of deidentified data, and the expectation of no more than minimal risk of harm. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
The incidence of IPLC and SPLC was identified through linkage to 2 state-level Surveillance, Epidemiology, and End Results registries, the Hawaii Tumor Registry and the California State Cancer Registry, through July 1, 2017. In this study, we excluded participants with missing smoking status (n = 3448 [1.6%]) in order to conduct a complete case analysis.
To accurately ascertain SPLC cases, we applied the most widely used clinical criteria by Martini and Melamed. 21 Per the Martini and Melamed criteria, new primary lung cancers (ie, SPLC) should meet at least 1 of the following conditions: (1) have different histology from that of the IPLC, (2) have at least 2 years of a disease-free interval from the time of IPLC diagnosis, or (3) arise in distinct lobes or lungs with no evidence of common lymphatics and extrapulmonary metastases at diagnosis. 21 To reduce the potential misclassification of recurrent or metastatic cases as SPLC in registry data, we used only the first 2 definitions. 17 , 22
The study had 2 primary outcomes. The first was the cumulative incidence of IPLC and SPLC. To account for the competing risk of death, we applied the Aalen-Johansen estimator 23 - 25 to calculate the cumulative incidence of IPLC in the entire cohort and the cumulative incidence of SPLC among patients with IPLC. The Aalen-Johansen estimator is a nonparametric estimator of the cumulative incidence function for a disease of interest using time-to-event data under competing risk. 23 - 25 The cumulative incidence function is modeled separately for each cause and event (including a competing event) using the overall survival information and the history of events and censoring over time. This method estimates the cumulative incidence of the disease of interest by removing patients who experience a competing event from the risk set, recognizing that the event of interest can no longer happen after the occurrence of a competing event. The cumulative incidence was stratified by smoking history. 26
The second outcome was the standardized incidence ratio (SIR) by smoking history, calculated as the SPLC incidence (observed SPLC cases over the person-years of the patients with IPLC) divided by the IPLC incidence (observed IPLC cases over the person-years of the entire cohort), which follows the National Cancer Institute’s multiple primary-SIR (MP-SIR) approach. 27 The MP-SIR describes the ratio of the incidence of new primary cancer in a population with initial primary cancer vs the incidence of initial primary cancer in the general population. We used the MP-SIR to calculate the incidence of new primary lung cancer (ie, SPLC cases) among patients who already had an IPLC diagnosis (numerator) compared with the incidence of new primary lung cancer (ie, IPLC cases) in the general population (denominator). We can then interpret the resulting MP-SIR estimate to be the comparative level of incidence for new primary lung cancers among patients already diagnosed with IPLC vs those without IPLC (ie, the general population). The 95% CI of the SIR was calculated using Byar approximation according to the MP-SIR method. 27 Given the common and error-prone issue of interpreting SIRs compared with risk ratios (eg, hazard ratio, relative risk ratio), 28 we included a comparative result as well as practical guidelines in the eMethods in Supplement 1 .
All analyses were performed using R, version 4.3.1 statistical software (R Project for Statistical Computing) with the prodlim package for computing cumulative incidence with the presence of competing events. Data were analyzed from July 1, 2022, to January 31, 2023.
As sensitivity analyses, we conducted subgroup analyses by sex due to the demonstrated sex-based differential incidence of IPLC 29 and smoking prevalence. 30 , 31 Additional subgroup analyses were performed among patients with early-stage IPLC (ie, localized and regional stages based on the SEER summary stage) who may exhibit longer survival with longer time at risk of developing SPLC, 32 as well as among patients with the most common IPLC histology (ie, adenocarcinoma) for those without a smoking history. 3 In addition, we conducted Poisson regression analyses to evaluate the SIR differences by smoking status, adjusting for covariates (ie, IPLC stage and histology). We used the Bonferroni method to adjust for multiple testing for 6 comparisons across sex and IPLC stage and histology, with a 2-sided significance level of P = .008 ( P = .05 / 6).
Among the 211 414 participants in MEC, the overall proportion of never-smokers was 44.7% compared with 55.3% of ever-smokers at enrollment ( Table ). Additional baseline characteristics of MEC have been previously reported. 20 Of MEC participants, 7161 (3.96%) developed IPLC over 4 038 007 person-years. Of these participants, the mean (SD) age at cohort enrollment was 63.6 (7.7) years, 4031 (56.3%) were male, and 3131 (43.7%) were female. By race and ethnicity, the cohort diagnosed with IPLC consisted of 1852 participants (25.9%) who were African Americans, 1633 (22.8%) Japanese American, 1005 (14.0%) Latino, 578 (8.1%) Native Hawaiian, 1714 (23.9%) White, and 379 (5.3%) other race or ethnicity (Chinese, Filipino, Korean, or unspecified). In this IPLC population, 163 (2.28%) developed SPLC over 16 470 person-years, 6315 (88.1%) had a smoking history, 2864 (40.0%) had adenocarcinoma, and 2837 (39.6%) had early-stage lung cancer at diagnosis ( Table ).
The overall 10-year cumulative incidence of IPLC in the entire cohort was 2.40% (95% CI, 2.31%-2.49%) among those who ever smoked, which was 7 times higher than the rate for those who never smoked (0.34%; 95% CI, 0.30%-0.37%) ( Figure 1 A). In contrast, the 10-year cumulative incidence of SPLC following IPLC diagnosis remained as high among patients with IPLC who never smoked (2.84%; 95% CI, 1.50%-4.18%) as those who ever smoked (2.72%; 95% CI, 2.24%-3.20%) ( Figure 1 B).
The overall SIR (ie, the ratio of SPLC to IPLC incidence) showed that the SPLC incidence (962.57 per 100 000 person-years) among patients with IPLC was 5.47 times higher than the IPLC incidence in the entire cohort (175.88 per 100 000 person-years) (SIR, 5.47; 95% CI, 4.67-6.38) (eTable 1 in Supplement 1 ). However, this SIR was substantially elevated (14.50; 95% CI, 8.73-22.65) among never-smokers (whose SPLC incidence was nearly as high as among ever-smokers despite their low IPLC incidence) compared with ever-smokers (3.50; 95% CI, 2.95-4.12) (eTable 1 in Supplement 1 ). We conducted a sensitivity analysis stratified by sex, given the differences in IPLC incidence 29 and smoking prevalence 30 , 31 by sex. The overall SIR among women (6.30; 95% CI, 4.99-7.85) was slightly higher than among men (4.69; 95% CI, 3.75-5.80) due to lower IPLC incidence among women, but the SIR difference by smoking history was similar across sexes (eTable 2 in Supplement 1 ; Figure 2 ). Given the high incidence of SPLC among patients with early-stage IPLC (ie, localized and regional), 14 , 22 we conducted a subgroup analysis for those diagnosed with early-stage IPLC. The results were consistent with the primary analyses, with a broader gap in the SIR by smoking history due to a lower incidence of early-stage IPLC among never-smokers (eTable 3 and eFigure 1 in Supplement 1 ). Consistent results were also observed in the subgroup analysis of patients with IPLC of adenocarcinoma histology, which is the most common histologic subtype among never-smokers (eTable 4 and eFigure 2 in Supplement 1 ). Sensitivity analysis using Poisson regression after adjustment for covariates showed similar results, producing higher SIR estimates due to the adjusted survival based on IPLC stage and histology (eTable 5 in Supplement 1 ).
In this cohort study, we examined the patterns of SPLC incidence among lung cancer survivors who never vs ever smoked. The findings suggest that the incidence of SPLC remains as high after IPLC diagnosis among patients who never smoked as in those with a smoking history. We used data from MEC, a large, ethnically diverse cohort from California and Hawaii with a long follow-up that would allow the detection of IPLC through SPLC. When examining SPLC risk by smoking history in subgroups stratified by sex, early-stage IPLC, and adenocarcinoma histology, our results remained consistent.
Though still poorly understood, interest in risk factors for lung cancer among never-smokers has risen as smoking rates have decreased over the past decades. Radon, 5 secondhand smoke, 6 and occupational exposures (eg, asbestos) 33 have each been associated with risk of IPLC among individuals without a smoking history, among other risk factors. 2 , 10 , 34 , 35 Additionally, a particularly high incidence of IPLC has been shown in never-smoking Asian women, 36 , 37 which may be relevant to this study given the racial diversity of the MEC. However, we were unable to obtain robust SIR estimates in our exploratory analysis stratified by sex and race combined due to a limited number of SPLC cases in the subgroup.
With therapeutic improvements and screening guidelines for IPLC, the number of SPLC cases among lung cancer survivors has gradually increased, but understanding of the risk factors for SPLC is still nascent. Although prior studies have identified tobacco smoking, 14 IPLC stage and histology, 17 , 18 or surgical resection for IPLC 15 as potential risk factors for SPLC, little is known about risk factors specific to never-smokers. Casal-Mouriño et al 32 suggested that median survival is longer among never-smokers than ever-smokers, which could increase the time at risk for SPLC for patients without a smoking history and may contribute to the high incidence of SPLC comparable to those with a smoking history. Other potential risk factors for SPLC may include underlying genetic susceptibility of the host, 2 environmental factors, 2 and long-term effects of cancer treatment. 38 Of note, the recent treatment landscape of lung cancer evolved with the introduction of targeted therapies and immunotherapy, and the long-term effects of these novel therapies on SPLC have not been studied and warrant future investigation.
This study uncovers the elevated incidence ratio of SPLC vs IPLC among never-smokers and underscores the need for tailored surveillance strategies for individuals at high risk for SPLC, including never-smokers. While the risk of recurrence is highest in the first 2 to 4 years after curative treatment, 39 , 40 SPLC exhibits a sustained risk for more than 10 years. 39 , 41 The longer survival of never-smokers with IPLC may translate into an even longer time at risk for SPLC, thus requiring long-term follow-up. Current American Society of Clinical Oncology 42 and National Comprehensive Cancer Network 43 guidelines recommend low-dose computed tomography scans annually after 2 to 3 years following curative treatment for surveillance of secondary malignancies, but the optimal screening strategy is unclear beyond 5 years since IPLC diagnosis. As randomized clinical trials are unlikely in this setting where stopping screening or screening less than annually may result in potential harm, alternative novel methods such as trial emulation and simulation studies that leverage large health care utilization and outcomes data sets may be critical next steps to fill the evidence gap.
To our knowledge, this study is among the first to evaluate the pattern of SPLC incidence by smoking history and to show a high incidence of SPLC among never-smokers using a population-based prospective cohort (MEC). Thus, this study directly addressed the lack of smoking status as a readily available variable in other large data sources, such as the Surveillance, Epidemiology, and End Results registry, which has prevented long-term IPLC and SPLC studies at the population level. Moreover, the racial and ethnic diversity of this data set increases the generalizability of the results from this study to a wider US demographic. Importantly, the use of large, prospective data with long-term follow-up from MEC, which followed through both IPLC and SPLC diagnoses, enabled an integrated analysis of SIR based on the use of a single large sample of the same individuals and allowed for subgroup analyses that showed consistent results regardless of sex, initial cancer stage of diagnosis, and IPLC histology.
This study also has several limitations. First, the SIR estimates were not age standardized as we followed the current practice of the MP-SIR approach. 27 Therefore, there is a potential bias because the exposure time after IPLC may be at older ages and later in the calendar year than the exposure time before IPLC. Future methodological studies should consider age-standardized incidence. Second, we were unable to account for the long-term effects of novel therapies, such as targeted therapy and immunotherapy, as the time span of our data did not overlap with when these therapies were commonly used (after 2016). Thus, future studies should investigate the late effects of novel treatments using a more contemporary data set. Third, baseline smoking data were used in this study, which did not capture changes in smoking behavior after IPLC diagnosis. However, because the likelihood of starting to smoke following an IPLC diagnosis is minimal among never-smokers, smoking status (never vs ever smoking) is not expected to change after baseline. Nevertheless, smoking cessation following an IPLC diagnosis could reduce the cumulative incidence of SPLC in the long term, and SIR differences by smoking history may be larger than estimated if smoking cessation is considered.
The findings of this cohort study show that the incidence of SPLC is as high among never-smokers as among those with a smoking history after an IPLC diagnosis, resulting in a substantially higher SIR among never-smokers than ever-smokers. While the reasons behind this observation are still poorly understood, the higher incidence of SPLC among never-smoking patients could be attributable to the longer survival reported in prior studies. Our findings highlight the critical need to identify risk factors for SPLC among never-smokers and to develop a targeted surveillance strategy for this patient population.
Accepted for Publication: October 5, 2023.
Published: November 15, 2023. doi:10.1001/jamanetworkopen.2023.43278
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Choi E et al. JAMA Network Open .
Corresponding Author: Summer S. Han, PhD, Quantitative Sciences Unit, Department of Medicine, Department of Neurosurgery, Stanford University School of Medicine, 3180 Porter Dr, Office 118, Stanford, CA 94304 ( [email protected] ).
Author Contributions: Drs Han and Choi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Choi and Ms Su contributed equally as co–first authors.
Concept and design: Choi, Aredo, Stram, Wakelee, Han.
Acquisition, analysis, or interpretation of data: Choi, Su, Wu, Neal, Leung, Backhus, Lui, Le Marchand, Liang, Cheng, Han.
Drafting of the manuscript: Choi, Su, Neal, Han.
Critical review of the manuscript for important intellectual content: Choi, Su, Wu, Aredo, Leung, Backhus, Lui, Le Marchand, Stram, Liang, Cheng, Wakelee, Han.
Statistical analysis: Choi, Su, Stram, Liang.
Obtained funding: Choi, Han.
Administrative, technical, or material support: Aredo, Neal, Leung, Le Marchand, Cheng, Wakelee.
Supervision: Backhus, Wakelee, Han.
Conflict of Interest Disclosures: Ms Su reported a summer internship from ZS Associates, Inc outside the submitted work. Dr Neal reported receiving personal fees and grants from the American Society of Clinical Oncology outside the submitted work. Dr Backhus reported serving on advisory boards for AstraZeneca and Genentech outside the submitted work. Dr Lui reported receiving personal fees from Intuitive Surgical and grants from Intuitive Foundation, Centese, and Auspex outside the submitted work. Dr Wakelee reported receiving grants paid to her institution from Arrys, Bayer, AstraZeneca, Bristol Myers Squibb, Clovis, Genentech/Roche, Merck, Novartis, SeaGen, Xcovery, and Helsinn and personal fees for data safety and monitoring committee service from Mirati outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by grants 4R37CA226081 and 1R01CA282793 from the National Institutes of Health and the Stanford Cancer Institute, a National Cancer Institute–Designated Comprehensive Cancer Center.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: The authors thank the Multiethnic Cohort Study participants for their contributions to this study and the research team for collecting, harmonizing, and sharing the data for research use.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

‘Here’s How I Knew I Had Lung Cancer’: One Patient’s Story with Relatively Low Risk Factor
Lung cancer , encompassing both small cell and non-small cell types and affecting men and women almost equally, stands as the second most common cancer in the United States. According to the American Cancer Society , the year 2024 is expected to see approximately 234,580 new cases and about 125,070 deaths due to lung cancer.
Predominantly diagnosed in those aged 65 and older, lung cancer is the leading cause of cancer death in the country, responsible for nearly 20% of all cancer fatalities. Each year, lung cancer claims more lives than colon, breast, and prostate cancers combined.
However, there's a silver lining. The incidence of lung cancer is on a decline, partly due to the decrease in smoking rates and advancements in early detection and treatment. Says Aaron Mansfield, MD, a Mayo Clinic lung cancer oncologist: "Although tobacco use is clearly the largest risk factor to develop lung cancer, there are many other risk factors, and risk factors we don't know. All you need to be at risk of developing lung cancer is a lung." Awareness of risk factors, such as exposure to secondhand smoke, radon gas, asbestos, and having a family history of lung cancer, is also crucial.
Despite the importance of early detection, lung cancer symptoms often appear only after the cancer has advanced. Dr. Mansfield explains one of the challenges that may be to blame for this: "Many of my patients have been diagnosed as having pneumonia. They've received many rounds of antibiotics that were not beneficial. They make their way to us when a mass that was thought to be pneumonia on a chest X-ray never improved."
Key symptoms of lung cancer to watch for include:
- shortness of breath
- persistent cough
- coughing up blood (hemoptysis)
- unexplained weight loss
- loss of appetite
- headaches (or other nervous system changes)
Screening also plays a pivotal role in combating lung cancer. Annual low-dose CT scans have been proven to save lives and are recommended for high-risk patients. (Data from the CDC in 2023 stated that cigarette smoking is the number-one risk factor for lung cancer, linked with 80% to 90% of all lung cancer deaths.)
Following recent updates, the US Preventive Services Task Force now suggests screening should start at age 50 for those with a 20-pack-year smoking history, broadening the net to catch more cases earlier. A "pack year" is a term used to describe the amount of tobacco a person has smoked over time, calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked. For smokers, quitting now can significantly reduce the risk of lung cancer.
Ahead, you'll read the personal story of Ron Barnett, a 65-year-old Philadelphia native, who shares his journey from a late-stage lung cancer diagnosis to finding stability and hope through a clinical trial. Ron shared that he smoked "occasionally" in his late teens to early twenties, but quit when he got married. It so happens that Barnett's home state of Pennsylvania is demonstrated to be one region with the highest radon rates, which some experts say is the second-highest lung cancer risk factor. To learn more about which areas around the country may be most vulnerable to radon exposure, read up on a recent radon study .
Get The Healthy by Reader's Digest newsletter
Here's how I knew I had lung cancer
By Ron Barnett, as told to Dr. Patricia Varacallo, DO
It's a strange thing, getting older. For awhile you might shrug off every ache and pain, but hit your early sixties, and suddenly you're keeping a ledger on every little twinge asking yourself if it's the big one. But lung cancer? That thought hadn't even crossed my mind.
Walking into the emergency room that brisk autumn morning in 2019, I was convinced the sharp pains in my chest were heralding a heart attack. You see, I've been a fighter all my life—raised in a tough neighborhood, served a stint in the military, and even beat a nasty case of pneumonia in my fifties—but nothing could've prepared me for the battle that lay ahead. The ER visit was supposed to be a quick in-and-out precaution to ensure my ticker was still ticking right. However, the doctors, after a flurry of tests and scans, found something unsettling—a spot on my liver. "It's concerning," they said, recommending a closer look.
That revealed a truth I wasn't ready to face: Stage 4 non-small cell lung cancer. Stage 4 is the most advanced type of cancer and usually means the cancer has spread, or "metastasized," to another organ. In my case, it had spread to my liver. A quick online search suggested my chance of surviving three months was maybe only around 25%. Fortunately, advances in science and medicine were in my favor.
But when I first heard those words, the room spun. The thought of "cancer" was a shock. My wife stood by my side, and together, we navigated some swift and fierce emotions.
Smokers Who Take This Vitamin Could Be at Higher Risk for Cancer
Treatment after I knew I had lung cancer
After the diagnosis sank in, I felt a kind of determined grit take over. The doctor briefed me on my battle plan: A three-drug chemotherapy regimen. It would be brutal, the doctor warned.
Let me tell you, chemo is no walk in the park. It's a beast unlike anything I'd faced before, but deep down, I understood it was my ticket through this storm.
But just as I was getting the hang of this new, unwelcome routine, the treatments began to falter. My doctor advised me that it seemed my body was no longer responding. The cancer was outpacing the chemo, and my options were dwindling fast.
11 Things About Lung Cancer Doctors Wish You Knew
That's when my doctor, seeing the desperation in my eyes, suggested a new course of action—a clinical trial. The uncertainty of it was terrifying— But what do I have to lose? , I remember thinking. I'd be lying if I said I wasn't scared, but I hung onto hope in this unknown.
The clinical trial included some new medications, regular monitoring, and ongoing doctors appointments...but slowly, almost imperceptibly, the tide began to turn. My cancer, this relentless force that had upended my life, started to show signs of stability. Three years have passed since then, and my cancer remains stable. It's a precarious peace, but I'll take it over the alternative any day.
I know I am one of the lucky ones, and I don't take that for granted. This journey has been anything but easy, but it's taught me more about life, resilience, and the importance of hope than I could've ever learned otherwise.
The Best Foods for Healthier Lungs, from Pulmonology Doctors and a Dietitian
To anyone going through a similar diagnosis, let me say this: Knowledge is power. Research your condition, understand your options, and don't be afraid to ask questions. A skilled medical team can make all the difference in the world, but so can your own understanding of what you're facing—and clinical trials? I know not every patient can say this, but in my case I found it was the lifeline I was looking for. Maybe that's a benefit of having a type of cancer that affects so many other people: With resources being allocated toward research, doctors are doing so much to make progress with new treatments these days.
I share my story not for sympathy but in the hope that it might light a path for someone else. Stage 4 lung cancer is not an easy diagnosis to live with, but it's not the end of the road. With the proper treatment, a great medical team, and the courage to explore new options, you can find a way to keep fighting.
For more inspiring stories of diagnoses and recovery, subscribe to The Healthy by Reader's Digest newsletter and follow The Healthy on Facebook and Instagram . Keep reading:
- "Here's How I Knew I Had Pancreatic Cancer": One Survivor's Story After Years of Growing Clues
- Colon Cancer and Poop: What to Watch For, According to a Colorectal Surgeon
- I Drank Tart Cherry Juice Every Day for a Week-Here's What Happened
- Watching Too Much TV Raises Your Risk of This Bathroom Issue, Says New Study
The post ‘Here’s How I Knew I Had Lung Cancer’: One Patient’s Story with Relatively Low Risk Factor appeared first on The Healthy .

Journal of Materials Chemistry C
Eu 3+ /tb 3+ -modified cd( ii ) coordination polymers for effective detection of uric acid and lung cancer biomarker n -acetylneuraminic acid †.

* Corresponding authors
a Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng, People's Republic of China E-mail: [email protected] , [email protected]
b School of Pharmacy, Liaocheng University, Liaocheng, People's Republic of China
Nowadays, lung cancer is a severe health burden worldwide and the major challenge faced in this disease is that more than half of the patients are diagnosed in later stages. The measurement of N -acetylneuraminic acid (NANA) levels, an important biomarker, could serve as a useful indicator for the early diagnosis of this disease. In this work, 1D coordination polymer Cd-CP2 was obtained and explored as a luminescence probe for detecting the urinary biomarker uric acid (UA) via a quenching effect. The presence of free carboxylic groups in the coordination polymer leads to the formation of Eu 3+ /Tb 3+ -functionalized samples, named Eu 3+ @Cd-CP2 and Tb 3+ @Cd-CP2 , via post-synthesis modification (PSM). Both of these samples were able to sense NANA via a ratiometric effect with the enhancement of ligand-centered peaks and quenching of rare earth peaks. The luminescence probe has several appealing merits, including a high selectivity, excellent sensitivity, low detection limit (0.89 μM for Eu 3+ @Cd-CP2 and 0.19 μM for Tb 3+ @Cd-CP2 ), and fast response time (less than 2 min). Furthermore, these fluorescent materials were explored as logic gates and test papers for potential application.

- This article is part of the themed collection: Journal of Materials Chemistry C HOT Papers
Supplementary files
- Supplementary information PDF (6531K)
- Crystal structure data CIF (791K)
Article information
Download citation, permissions.
Eu 3+ /Tb 3+ -modified Cd( II ) coordination polymers for effective detection of uric acid and lung cancer biomarker N -acetylneuraminic acid
H. Li, Y. Chen, H. Zhao, H. Zou, H. Yan, J. Lu, H. Hao, J. Dou, Y. Li and S. Wang, J. Mater. Chem. C , 2024, 12 , 3141 DOI: 10.1039/D3TC03775D
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author, advertisements.

IMAGES
VIDEO
COMMENTS
Lung cancer remains a devastating diagnosis to receive. The goal of this thesis was to better understand the impact of lung cancer and study how to integrate palliative and supportive care.
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable ...
Introduction. Lung cancer (LC) has been the most common cancer and the leading cause of cancer-related death worldwide for several decades. There were an estimated 2,093,876 new cases of LC and 1,761,007 LC-related deaths worldwide in 2018, representing 11.6% of all new cancer diagnoses and 18.4% of all cancer-related deaths. 1 The incidence of LC varies considerably by geographic region and ...
This thesis will be defended in public on Wednesday 24 February 2016 at 11:00 hrs by Ali Saber Hosseinabadi born on 11 September 1981 in Rasht, Iran . Supervisors ... Lung cancer is a result of multiple genetic alterations that accumulate during life21. In 1982, a human gene with transforming activity was identified in a lung ...
A collection of material about the ALCHEMIST lung cancer trials that will examine tumor tissue from patients with certain types of early-stage, completely resected non-small cell lung cancer for gene mutations in the EGFR and ALK genes, and assign patients with these gene mutations to treatment trials testing post-surgical use of drugs targeted against these mutations.
The 100 most cited articles in lung cancer are listed in Table 1, arranged in descending order according to the number of times cited. The number of citations ranged from 7751 to 889, and the mean number of citations per article was 1879.82 ±1264.78 (range: 7751-889). We found that the most cited article (times cited: 7751) on lung cancer ...
In this thesis, we focus primarily on patients with lung cancer by trying to better understand the impact of this disease and ... Lung cancer is the leading cause of cancer-related mortality in the majority of Western countries (Figure 1).1,3 In the United States alone, approximately 235.000 new patients are diagnosed
Lung cancer has four common types: lung nodules, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), and mesothelioma; the most common of which is non-small cell lung cancer [6]. It is important that research and modeling is done on lung cancer in an attempt to better understand and counteract this disease. 1.1 A549 Cells
The pipeline consists of 2 steps, detection and classification. First, a 3D CNN is built to detect suspicious nodules and predict bounding boxes. 3D small patches of size 128x128x128x1 are extracted from the scans and input to the next work and patches going beyond the range of lung scans is padded with value 170.
4.1 Current challenges in lung cancer research 25 4.2 What is required to address the knowledge gaps in lung cancer research? 28 -Research methods and methodology 28 -Smoking and tobacco control 29 -Screening and treatment 30 -Sex and lung cancer risk: biological factors 33 5. Conclusion 34 6. References 35 7. Additional resources 43 ...
Lung cancer is the primary cause of cancer death worldwide, with 2.09 million new cases and 1.76 million people dying from lung cancer in 2018 1.Four case-controlled studies from Japan reported in ...
2. Adenocarcinoma vs. Squamous Cell Carcinoma. The 2015 World Health Organization (WHO) classification was recently modified based on newly identified molecular profiles and druggable genetic alterations in lung cancer [].In particular, the 2011 International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society, and the European Respiratory Society classification ...
Bradley, Stephen (2022) The role of imaging in the diagnosis of lung cancer in primary care. PhD thesis, University of Leeds. Abstract. Background Lung cancer is the leading cause of cancer death worldwide. The UK relies more heavily upon chest x-ray than many other high income countries. Little is known about the performance of the test, the ...
Lung Cancer Screening Program Revenue April 2016 verses 2017. A 2012 study found lung cancer screening to be one of the most cost-effective. screening tests available at about $11,000 per life-year saved, compared with $18,000 for. colon cancer screening, $31,000 for breast cancer screening, and $50,000 for cervical.
both limited-stage small cell lung cancer patients and extensive-stage small cell lung cancer patients throughout the study period. Lu et al. (2019) demonstrated that the average incidence of lung cancer was 59.0/100,000 people for the years from 1973 to 2015. Specifically, the incidence rate was at an apex in 1992 and then constantly declined.
Introduction. Lung cancer is the number one cause of cancer related mortality. Over the last decade, a greater understanding of the biology of lung cancer at the molecular level has led to the development of new and effective therapies resulting in improvement in overall survival (OS), mostly driven by advances in the treatment of non-small cell lung cancer (NSCLC) ().
approximately 2.09 million new cases and 1.76 million lung cancer-related deaths [2]. Lung cancer cases and deaths have increased significantly globally [2]. Approximately 85-88% of lung cancer cases are non-small cell lung carcinoma (NSCLS), and about 12-15% of lung cancer cases are small cell lung cancer (SCLC) [3]. Early lung cancer ...
The NCI-sponsored National Lung Screening Trial (NLST) showed that low-dose CT scans can be used to screen for lung cancer in people with a history of heavy smoking. Using this screening can decrease their risk of dying from lung cancer. Now researchers are looking for ways to refine CT screening to better predict whether cancer is present.
Lung cancer ranks as the second most common cancer worldwide. According to the International Agency for Research on Cancer, there were about 2.2 million new cases of lung cancer in 2020, accounting for 11.4% of cancer diagnoses. Lung cancer also causes 1.8 million deaths, accounting for 18.0% of all cancer deaths [1]. The heterogeneity of the ...
Lung cancer is the leading cause of cancer-associated deaths in the newlineworld, and often requires surgical intervention for tumour therapy. ... The thesis is also directed towards newlineproviding sterile, contactless, gesture-based, computer-aided guidance for newlineaccessing medical data during surgery. newlineThe quality of lung CT image ...
UCL Discovery - UCL Discovery
Moreover, NSCLC is going to be the topic of exploration for this thesis, and it consists of three groups that are identified based on the initial location of cancer. First, the most common type of lung cancer that emerges in the glandular cells on the outer part of ... lung cancer are "small cell carcinoma and combined small cell carcinoma ...
In an era of expanding perioperative approaches for resectable non-small-cell lung cancer, new data demonstrate that dual neoadjuvant immunotherapy targeting PD-1 and LAG-3 is feasible; future ...
Lung cancer remains the leading cause of cancer-related mortality in the US, causing more deaths than breast, prostate, and colon cancer combined. 1 While smoking is the predominant risk factor, approximately one-quarter of patients worldwide develop lung cancer without a smoking history. 2-4 Although the risk factors for lung cancer among ...
My thesis work in the lab of Danwei Huangfu, ... Validating AlphaMissense's predictions in two cohorts of patients with lung cancer, we identified mutations in KEAP1 and SMARCA4 that are associated with poorer survival, suggesting their significance in cancer progression. This research validates computational tools for real-world outcomes ...
Lung cancer, encompassing both small cell and non-small cell types and affecting men and women almost equally, stands as the second most common cancer in the United States.According to the ...
Nowadays, lung cancer is a severe health burden worldwide and the major challenge faced in this disease is that more than half of the patients are diagnosed in later stages. The measurement of N-acetylneuraminic acid (NANA) levels, an important biomarker, could serve as a useful indicator for the early diagn Journal of Materials Chemistry C HOT Papers
2023 Oral Examination Committee Member, OHSU, Ph.D. Thesis, Breanna Caruso 2017 - 2020 Undergraduate Research Mentor and Thesis Advisor, 2 students, Jacks Laboratory ... Lung Cancer Discovery Award (PI: Burger) 07/01/2023-06/30/2025 Source: American Lung Association