
U.S. Government Accountability Office

Federal Research and Development: Funding Has Grown since 2012 and Is Concentrated within a Few Agencies
Innovation is critical to U.S. competitiveness, prosperity, and security. In the last 10 years, the federal government has increased funding for research and development (R&D)—investing $179.5 billion in FY 2021.
DOD and the Department of Health and Human Services received 77% of the FY 2021 funding. COVID-19 stimulus funding led to large R&D increases for HHS. For example, an HHS agency that helps develop vaccines saw increased spending from $736 million in FY 2019 to $16 billion in FY 2020.
Some funding supports multi-agency initiatives in complex areas of strategic national importance—such as nanotechnology and artificial intelligence.
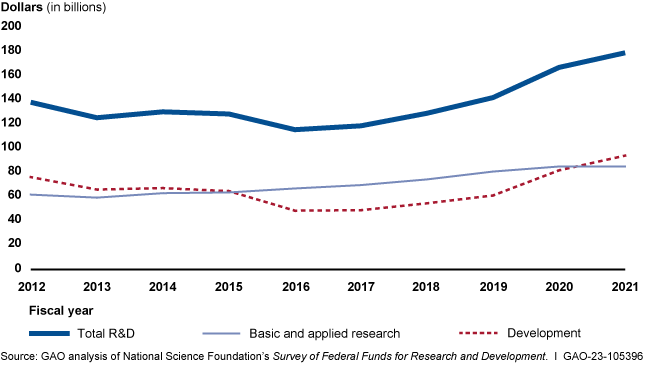
Federal Research and Development Investments, FYs 2012-2021
What GAO Found
Federal research and development (R&D) funding has increased since 2012—most recently because of COVID-19 stimulus funding. Five agencies obligated the majority of federal R&D funding with the Departments of Defense (DOD) and Health and Human Services (HHS) accounting for nearly 80 percent in fiscal year 2021 (see figure). HHS has mainly funded research, while DOD mainly funds development. However, HHS has become a major funder of development in recent years because of COVID-19 stimulus funding. HHS averaged less than 1 percent in development funding through fiscal year 2019 but reported 37 percent of its R&D obligations were for development in fiscal year 2021. Of the estimated $179.5 billion in federal R&D obligations in fiscal year 2021, about two-thirds went to organizations outside the federal government. In fiscal year 2021, industry, universities, and colleges received the majority of these external R&D obligations—almost $90 billion.
Federal Research and Development Obligations, Fiscal Year 2021
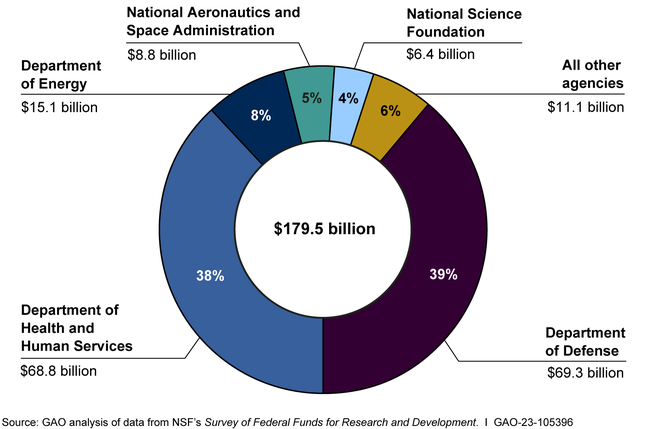
Note: FY 2021 data are estimates provided by federal agencies to the National Science Foundation.
Federal funding also includes four multi-agency initiatives in areas identified as having long-term national importance, such as quantum information science and nanotechnology. These initiatives coordinate activities in areas that are too broad or complex to be addressed by one agency alone. For example, more than 60 agencies participate in an initiative on network and information technology, which includes investments in artificial intelligence and machine learning. Not all participating agencies contribute funding to such initiatives. Funding for these initiatives increased over the previous decade, and accounted for roughly $14 billion in fiscal year 2020, just under 9 percent of the total federal R&D budget.
Why GAO Did This Study
Scientific and technological innovation are critical to long-term U.S. economic competitiveness, prosperity, and national security. The U.S. has long been a global leader in advancing the frontiers of science and technology. Increased competition from other countries has led some experts to express concern that the U.S. may be losing its competitive edge in certain technologies. Agencies are investing in various R&D initiatives, including those that are of strategic national importance, such as network and information technology, nanotechnology, quantum information science, and global environmental changes.
This report describes (1) trends in federal R&D funding over the last 10 years and (2) the funding and organization for selected multi-agency R&D initiatives, among other objectives.
To address these objectives, GAO analyzed data published by the National Science Foundation on annual R&D expenditures and examined Office of Management and Budget (OMB) data. GAO also reviewed agency documentation and collected written responses to structured questions on federal R&D from the Chief Financial Officer or budget office from the five agencies that fund most R&D.
In addition, GAO interviewed officials from OMB and the Office of Science and Technology Policy, including the Directors of the National Coordination Offices for selected multi-agency R&D initiatives, which are coordinated under the auspices of the National Science and Technology Council.
For more information, contact Candice N. Wright at (202) 512-6888 or [email protected] .
Full Report
Gao contacts.
Candice N. Wright Director [email protected] (202) 512-6888
Office of Public Affairs
Chuck Young Managing Director [email protected] (202) 512-4800

- Become a Member
An Introduction to U.S. Federal Funding for Healthcare Innovation

September 8, 2021 – By Amrika Ramjewan, Principal Strategist – Mayo Clinic Innovation Exchange
Each year, U.S. government agencies with extramural research and development (R&D) budgets invest in federal funding programs designed to stimulate technological innovation and foster entrepreneurial activity. Coordinated by the U.S. Small Business Administration (SBA), these federal funding programs are accessible via a competitive award process through major research agencies such as the National Institutes of Health (NIH), Department of Defense (DoD), and National Science Foundation (NSF).
Entrepreneurs seeking to advance research and bring their innovations to market have the opportunity to compete for these funds and access a wealth of support from these agencies. Through 2019, over 179,000 awards have been granted totaling over $54.3 billion in investment by the federal government.
Jon Zurn, director of the Strategic Funding Office for Research at Mayo Clinic , spoke with the Exchange’s members about the variety of federal funding options available, how the non-dilutive granting process works, and the resources available to entrepreneurs.
Q: Can you share an overview of the types of federal funding programs available to entrepreneurs?
JZ: The U.S. Small Business Administration (SBA) coordinates non-dilutive, research innovation funding programs to assist entrepreneurs and small businesses with planning and conducting R&D activities and advancing their products and technologies through validation and commercialization. Two types of grants are available including the Small Business Innovation Research (SBIR) award and the Small Business Technology Transfer (STTR) award . These awards focus on R&D, stimulating technological innovation, and increasing private-sector commercialization of innovation derived from federal R&D funding.
SBIR funds are offered by 11 federal agencies and divisions within these agencies including the National Institutes of Health (NIH), Department of Defense (DoD), National Science Foundation (NSF) and the Environmental Protection Agency (EPA), among others. The funds are intended to assist small businesses with conducting principal investigator-led R&D on their own or with subcontractors, with the expectation that a majority of the work will be completed by the small business.
STTR funds are offered by five federal agencies, including the Department of Health and Human Services (HHS, and its constituents NIH, FDA, CDC, and ACL). The funds are intended to facilitate collaboration and foster technology transfer between small businesses and non-profit research institutions. The government recognizes that small firms often don’t have the research resources and infrastructure to complete early-stage R&D, and that there is tremendous value in partnering with large, research-intensive institutions to bring innovations to market.
Both awards are non-dilutive sources of funding, meaning that the government takes no interest or equity stake in your business. Recipients of these funds are expected to fulfill the reporting requirements laid out by the awarding federal agency, and all intellectual property is owned by the small business (except in special circumstances).
Q: What can the funds from these programs be used for?
JZ: SBIR and STTR grants are intended for performing R&D. Purchasing equipment, commercializing a technology that has already been developed, or pursuing a low-risk idea that requires capital will typically not be funded by these programs. Before applying, it’s best to consult with an agency’s program officer to make sure your idea meets the R&D criteria.
Award solicitations for these grants, called an Omnibus or Parent Announcement, are published by the NIH three times each year — in January, April, and September. Each participating NIH institute and center (I/C) has its own research priorities. It is important to understand how these priorities align with your projects. Additionally, targeted solicitations for specific needs may also be published, but these are not released on a regular cycle. Other federal agencies tend to post opportunities throughout the year.
Funding for each award is focused on distinct phases. In Phase I, the objective is to establish technical merit, feasibility and commercial potential prior to seeking Phase II funding. Phase I SBIR/STTR awards normally do not exceed $150,000 in total over six months (for SBIR), or over one year (for STTR) — with some exceptions.
In Phase II, the funding is based on the results achieved in Phase I, with the possibility of funding through Phase IIB. Phase II awards normally provide up to $1,000,000 in total over two years. However, exemptions may even be granted for approved research areas that may extend the award ceiling up to $1.7 million for Phase II.
Phase III research on the path of commercialization is not funded by SBIR/STTR. However additional, late stage development federal dollars are available through programs such as the NIH’s Commercialization Readiness Pilot (CRP).
Q: Who is eligible for these funds?
JZ: Small, for-profit business organizations that are U.S. concerns, and operating primarily in the United States with a U.S.-based location are eligible to apply for SBIR and STTR dollars. The small business, including its affiliates, must have no more than 500 employees, and must be more than 50% directly owned and operated by one or more individuals who are citizens or legal permanent residents of the United States. Small businesses that are subsidiaries of larger companies are not eligible.
However, if a small business is majority-owned by multiple venture capital operating companies (VCOCs), hedge funds, or private equity firms that each meet small business size criteria, it is eligible to apply for an NIH SBIR funding opportunity. These grants are also not designated for large institutions, universities, or non-profit organizations.
Foreign (non-U.S.-based) firms may access SBIR and STTR dollars through two avenues — either as a subcontractor to a U.S.-based firm, or by having a U.S. location where the work for which the funds being sought will be completed. That is, all grant dollars must be spent in the U.S. For example, if a foreign firm owned by a U.S. legal permanent resident were to receive a three-year grant for $100,000, all of the funds must be spent in the U.S. to complete the research.
For SBIR grants, subcontracting is limited to 33% of the total effort in Phase I of the project, and 50% of the total effort in Phase II. Also, the principal investigator (PI) leading the research must be employed by the small business seeking the funds. This means that the PI will be unable to work elsewhere during the project period, as more than 50% of their time must be spent in service to the small business.
For STTR grants, 40% of the work must be completed by the small business, and 30% by the collaborating research institution (RI). The remaining 30% may be completed by the small business, or outsourced to either the RI or another subcontractor. The PI may be primarily employed by either the small business or the RI. Co-investigators may be affiliated with either the small business, or the RI, or they may serve as consultants — however, this is dependent on any restrictions that may be set by the funder.
Any organization located in the U.S. that is a university, non-profit institution, or contractor-operated federally funded research and development center (FFRDC) is eligible to collaborate with small firms on STTR projects.
Q: What resources are available to entrepreneurs and small businesses interested in seeking funding through these programs?
JZ: There are a tremendous number of online resources , tutorials , as well as local SBA affiliates in every state. Many agencies provide applicant assistance programs for businesses interested in applying for SBIR and STTR grants, and technical support staff provide good, free advice — remember, they are there to help.
The National Institute of Allergy and Infectious Diseases (NIAID) publishes sample applications , as well as many useful templates for preparing proposals. Agencies such as the NIH and NSF offer Innovation Corps (I-Corps™) programs, which offer more in-depth support, including funding, mentoring, and networking opportunities on a team’s journey towards commercialization.
The realm of finding funding and development opportunities can seem complicated, but once you’re in it, the ecosystem is quite exciting, with many resources available to help you navigate.
Q: What breakthrough innovations in healthcare delivery or technology excite you most?
JZ: Artificial intelligence (AI) is already a burgeoning field and seems to be growing daily. It’s being applied in nearly every corner of biomedical research and healthcare, from mechanistic studies to improving staffing workflows. It’s also ripe for multidisciplinary collaboration, including small businesses with specialized skills or AI technologies.
Federal agencies are increasing their AI investments, including a plan to stand up an entirely new $6.5 billion office at NIH, the Advanced Research Projects Agency for Health (ARPA-H) . The goal is to build high-risk, high-reward capabilities (or platforms) to drive biomedical breakthroughs. This includes achieving viable products and market feasibility. Undoubtedly, small businesses will be welcome in this new arena.
I’m excited, too, about microbiome research, which is an interest area to not just the NIH, but 15 other federal agencies as well. The biome is another new frontier in medicine that we’re now learning plays a role in numerous diseases. Like AI, we’re finding application of microbiome research in a wide span of applications, including unlocking molecular secrets, developing biomarkers, creating therapeutics, and improving lifestyles.
This is such an exciting time to be involved in medical research.
Call for Healthcare Innovations
Are you developing an innovative healthcare technology? Contact the Mayo Clinic Innovation Exchange to learn how membership can help bring your company or your idea closer to patients.
- Open access
- Published: 18 February 2016
The 10 largest public and philanthropic funders of health research in the world: what they fund and how they distribute their funds
- Roderik F. Viergever 1 &
- Thom C. C. Hendriks 2
Health Research Policy and Systems volume 14 , Article number: 12 ( 2016 ) Cite this article
107k Accesses
100 Citations
198 Altmetric
Metrics details
Little is known about who the main public and philanthropic funders of health research are globally, what they fund and how they decide what gets funded. This study aims to identify the 10 largest public and philanthropic health research funding organizations in the world, to report on what they fund, and on how they distribute their funds.
The world’s key health research funding organizations were identified through a search strategy aimed at identifying different types of funding organizations. Organizations were ranked by their reported total annual health research expenditures. For the 10 largest funding organizations, data were collected on (1) funding amounts allocated towards 20 health areas, and (2) schemes employed for distributing funding (intramural/extramural, project/‘people’/organizational and targeted/untargeted funding). Data collection consisted of a review of reports and websites and interviews with representatives of funding organizations. Data collection was challenging; data were often not reported or reported using different classification systems.
Overall, 55 key health research funding organizations were identified. The 10 largest funding organizations together funded research for $37.1 billion, constituting 40% of all public and philanthropic health research spending globally. The largest funder was the United States National Institutes of Health ($26.1 billion), followed by the European Commission ($3.7 billion), and the United Kingdom Medical Research Council ($1.3 billion). The largest philanthropic funder was the Wellcome Trust ($909.1 million), the largest funder of health research through official development assistance was USAID ($186.4 million), and the largest multilateral funder was the World Health Organization ($135.0 million). Funding distribution mechanisms and funding patterns varied substantially between the 10 largest funders.
Conclusions
There is a need for increased transparency about who the main funders of health research are globally, what they fund and how they decide on what gets funded, and for improving the evidence base for various funding models. Data on organizations’ funding patterns and funding distribution mechanisms are often not available, and when they are, they are reported using different classification systems. To start increasing transparency in health research funding, we have established www.healthresearchfunders.org that lists health research funding organizations worldwide and their health research expenditures.
Peer Review reports
Approximately 40% of all health research in high-income countries is funded by public and philanthropic funding organizations [ 1 ]. These organizations play a central role in the development of new knowledge and products, particularly in areas that are not sufficiently profitable [ 2 ]. For example, the involvement of public and philanthropic funding organizations has been key in the development of new medical products to combat neglected diseases [ 1 , 2 ] and, since recently, these organizations are increasingly taking action to address the lack of development of new antibiotics [ 3 – 5 ].
Transparency on who the main funding organizations of health research are, on what they fund (their funding patterns) and on how they decide on what gets funded (their priority setting mechanisms and funding distribution mechanisms) can help funding organizations to synchronize their efforts, potentially preventing the duplication of research and improving collaboration on research priorities, and has various other strategic and practical benefits for funders [ 2 , 6 – 12 ]. Such transparency also allows for external evaluation of funding organizations’ portfolios and decision-making processes [ 7 , 13 ]. This is particularly important for public funding organizations, since they distribute public funds. For philanthropic funders, such accountabilities are less clear, but given the substantial impact of some of these funders on the global landscape for health research, it might be reasonable to make similar demands from this group of funders [ 14 , 15 ].
Although substantial insight has been created in recent years into countries’ expenditures on health research [ 1 , 16 – 20 ], there has been relatively little scrutiny of the funding patterns and mechanisms of individual funding organizations. Mappings of individual funding organizations’ expenditures on health research are often limited to one or several countries [ 7 , 10 , 21 – 26 ] or to a select group of diseases [ 25 , 27 – 29 ]. To increase the available information on major public and philanthropic funders of health research, we present a mapping in this article that had a simple target: to identify the 10 largest public and philanthropic funders of health research in the world, to report on what they fund, and on their mechanisms for distributing these funds (funding organizations’ priority setting mechanisms were beyond the scope of this study – see Limitations section for more detail).
Here, we outline the methods used to identify the 10 largest funding organizations of health research in the world, and to assess the funding patterns and funding distribution mechanisms of these organizations. A more detailed description of these methods is provided in Additional file 1 . All data were collected from November 4, 2013, to August 12, 2014.
Identifying the 10 largest funders of health research
Search strategy.
This study distinguished between four types of public and philanthropic health research funders: (1) public national or regional funders (excluding funders of official development assistance (ODA) and multilateral funders), (2) philanthropic funders, (3) ODA funders, and (4) multilateral funders. The mandate of the funding body did not need to be limited to funding health research. Funding organizations were identified through a search strategy that had several components: key funding organizations in the 20 countries with the highest spending on health research [ 1 ] were identified, membership lists of collaborative groups of funders (i.e. groups where major funders of health research collaborate on a global or regional level) were reviewed, publicly available lists of funding organizations that included annual spending on health research were searched, and data on Development Assistance for Health were used to identify key ODA funders. For every funder type, a specific search strategy was used to identify the largest funders of health research (Additional file 1 ). Private for-profit funding organizations were not included in our analysis; we only aimed to map public and philanthropic funders (private for-profit health research funders are mapped elsewhere [ 30 ]). Product development partnerships (PDPs) and other public private partnerships (PPPs) were also excluded because they are intermediate funding organizations, who are funded in turn by governments, philanthropies and the for-profit sector. Furthermore, we excluded single disease funders; although the majority of philanthropic funders of health research focuses on one disease [ 21 ], the largest philanthropic funders of health research tend to fund across multiple disease areas (with some exceptions [ 31 , 32 ]). We note that the annual health research expenditures of the largest PDP, PPP and single-disease funders that we are aware of are lower than the annual expenditures of the 10 largest public and philanthropic funders reported in this study (see Additional file 1 ). Finally, in two cases (the United States Department of Defense (US DoD) and the European Commission (EC)) we included both the overarching organization at its largest sub-organizations or sub-programmes, because of the substantial differences between the funding distribution mechanisms of these sub-organizations and sub-programmes.
To aid future analyses of this kind, we provide an overview of various sources that helped us identify the main public and philanthropic funders of health research globally in Additional file 2 .
Assessing health research expenditures
For all the funding organizations that followed from our search, publicly available data were collected on the organizations’ annual health research expenditures (from annual reports and websites). Data were collected for the most recent year available. When we were not able to find data on organizations’ annual expenditures in the public domain, we contacted funders to ask if they could provide us with their annual expenditures on health research.
Funding organizations differ on at least three aspects in terms of how they report their annual health research expenditures. First, expenditures can be reported as actual expenditures, commitments or budgets. Second, there can be differences in terms of what the expenditures cover. They can cover the organization’s total expenditures on health research excluding operational costs (for managing the funding organization), its total expenditures including operational costs, or its total overall turnover over a single fiscal year (this was only collected if the funding organization exclusively funded health research). Third, there can be differences in terms of the research areas that the reported expenditures pertain to: only health research, or broader categories such as health and biological research or life sciences research. For each funder we extracted data on annual health research expenditures in a step-wise manner, always reporting the actual expenditures excluding operational costs in the area of health research when possible. When these numbers were not available, we reported the next best available number, following the order in the categories provided above. We note that the data from the funding organizations in the top 10 all relate only to health research, all concern actual expenditures or commitments, and for all, except one, operational costs were excluded.
Training support and research education were not included in the overall amount for health research expenditures. In addition, for government ministries, we excluded two types of funding flows. First, when funding was provided by ministries to funding agencies for distribution, we included the funding for the funding agencies, but not for the ministries. Second, for government ministries, such as ministries of education or health, we excluded block funding to universities or hospitals (similar to other initiatives that have reported on health research funding flows [ 24 ]). For funding agencies, we did include institutional funding.
Finally, organizations’ expenditures were made comparable using methods by Young et al. [ 17 , 20 ]. To do so, we first deflated organizations’ expenditures in the national currency to the year 2013 using Gross Domestic Product deflators from the International Monetary Fund World Economic Outlook Database of April 2014 [ 33 ]. Second, we converted the inflation-corrected expenditures to US dollars using the World Bank Official exchange rates for the year 2013. As a secondary outcome, we calculated funding organizations’ health research expenditures as 2013 purchasing power parity-adjusted US dollars (these are not reported in this article, but are available on www.healthresearchfunders.org ) [ 17 , 20 ].
Assessing the funding patterns and funding distribution mechanisms of the 10 largest funders of health research
After the 10 largest funding organizations of health research were identified, data were collected on their funding patterns and funding distribution mechanisms. For each organization, data were collected on:
The funding mechanisms used to distribute funding: intramural funding or extramural funding. For extramural funding, we distinguished between project grants, ‘people grants’, programme grants, funding distributed to organizations and other extramural research funding. For project grants, data were collected to assess if the distribution was untargeted, targeted or highly targeted (for definitions see Additional file 1 ).
The amount of funding allocated to a list of 20 key health areas from the Global Burden of Disease classification [ 34 ].
Funding for operational expenditures was excluded.
Finally, we denoted whether funding organizations used a classification system to classify funding to various health areas and whether they reported statistics on funding for various research types (e.g. biomedical research, clinical research, epidemiological research or health systems research [ 35 ]) and recipient countries or regions.
All data were collected from online reporting databases, annual reports, official websites, or other information sources. After this, each funder was invited to participate in an interview. Before the interview, a document with collected data was made available to a representative of the funder. Before and during the interviews, representatives were asked to add, amend or confirm the data.
Identifying the 10 largest funding organizations of health research
Public and philanthropic funding organizations.
Our search identified 55 public and philanthropic funders that were candidates for being one of the 10 largest funders of health research in the world (Table 1 ), excluding ODA funders and multilaterals (we searched separately for these and report on them later). For 41 organizations, data on the organizations’ annual health research expenditures were available. For five of these organizations, this information was received through personal communications (not publicly reported). Fourteen funders did not provide figures about their annual health research expenditures. Often, these organizations were general funders of research and did provide overall expenditure data but not for health research specifically.
For the 10 largest funders, health research funding totalled to $ 37.1 billion, approximately 40% of all spending on health research globally by public and philanthropic sources [ 1 ]. The United States National Institutes of Health (NIH) contributed the largest part of this amount, with $ 26.1 billion in health research funding in 2013. The largest philanthropic funder was the Wellcome Trust ($ 909.1 million). The Wellcome Trust and the Howard Hughes Medical Institute (HHMI) were the only two philanthropic funders among the 10 largest funders of health research; the other eight organizations were public funding bodies. All 10 funders came from Northern America, Europe or Oceania. The largest Asian funding organization identified was the National Natural Science Foundation of China (NSFC) ($ 621.3 million), the largest funder from Latin America and the Caribbean was Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) from Argentina ($ 184.4 million), and the largest African funder was the South African Medical Research Council (SA MRC) ($ 63.2 million).
ODA-agencies and multilaterals
The expenditures of ODA-agencies and multilaterals on health research were substantially smaller than the expenditures of the largest public and philanthropic funding organizations (Tables 2 and 3 ). The largest funder of health research through ODA was USAID ($ 186.4 million) and the largest multilateral funder was WHO ($ 135.0 million).
Assessing the funding patterns and funding distribution mechanisms of the 10 largest funding organizations of health research
Funding mechanisms used to distribute funding.
There was considerable diversity in organizations’ funding distribution mechanisms (Table 4 ). Five funders funded research fully extramurally, five allocated at least a proportion of their funding to intramural research institutes, and one funder, the Institut national de la santé et de la recherche médicale (Inserm), funded research (almost) exclusively intramurally (total is 11 because for the EC and the US DoD we analysed the sub-organizations or sub-programmes: the US Congressionally Directed Medical Research Program (CDMRP), the Health theme of the EC FP7 Cooperation programme and the European Research Council (ERC)).
Of the 10 funding organizations that provided extramural funding, for six, the main mechanism for extramural funding distribution was the allocation of funding through untargeted competitive project or investigator grants (often, there were also some smaller programmes that used a more targeted distribution). Two funders, the Health theme of the European Commission FP7 Cooperation programme and the US CDMRP, used a more targeted approach and issued calls under prioritized areas. Funders also made use, in varying degrees, of highly targeted funding schemes, such as research contracts, tenders or prizes, but this was never the dominant form of funding distribution. The last two funders, the United Kingdom Medical Research Council (MRC) and the Deutsche Forschungsgemeinschaft (DFG), used a mixed approach to allocate funding, with substantial contributions made through different funding distribution mechanisms. Lastly, the funding model of the NIH and the untargeted part of the MRC deserve separate mentioning because, although they adhered largely to an untargeted model and research funding was available for all areas of health research, the amounts of funding available for various broad research areas were earmarked (in the case of the NIH, for example, through budgets for the NIH institutes). This differs from targeted approaches, where not all areas have to be funded and the prioritization is often more specific, but it is also not completely untargeted.
Finally, most funders mainly dispensed funding via project grants, with smaller programmes that provide grants to excellent individual researchers. However, others put more focus on individual excellence. The HHMI has traditionally been a proponent of such people-focused funding. Since recently, other funders, such as the Wellcome Trust and the NIH, are increasingly making use of ‘people grants’ as well [ 36 ].
Funding patterns towards diseases
The funding organizations’ research expenditures towards 20 specific health areas are shown in Table 5 . We could report data for at least one health area for seven funders. However, as the table makes clear, these data were often not available.
Funding patterns varied, with some funders showing preferences for investing in non-communicable over communicable diseases and others showing the opposite. For example, the NIH spent less on infectious disease research in total than on cancer research alone, while the Wellcome Trust spent 14 times more on infectious disease research than on cancer research. Similar variations arose when comparing more specific disease areas within the non-communicable or communicable diseases. For example, the NIH spent almost three times more on cancer research than on cardiovascular research while the EC under the FP7 programme spent roughly equal amounts on both, and while HIV/AIDS funding comprised more than half of the infectious disease research funding at the US NIH, it comprised less than 10% of that funding at the Australian National Health and Medical Research Council (NHMRC).
Six funders used classification systems to classify their funding to health areas (Table 6 ); five different classification systems were used by these funders (the two funders from the United Kingdom used the same system). Besides using different categories for health problems, these systems also varied on other aspects, such as who enters the data (e.g. the researcher or a specialist employed by the funder) and whether grants can be indexed as belonging to one or multiple health problems. Seven funders reported amounts of funding allocated to various research types and the same seven reported how much funding was allocated to various recipient countries or regions.
In this article, we have identified the 10 largest funding organizations of health research globally and shed more light on their funding distribution mechanisms and funding patterns. Two main conclusions can be drawn from this mapping of influential funders of health research.
Differences between funding organizations: the need for more evaluation of funding distribution models
First, there is considerable diversity between funding organizations in terms of what they fund and how they distribute those funds. This begs the question: do some funding distribution models have more impact than others? The impact of different approaches to funding health research is regularly discussed in the literature, for example, for intramural versus extramural funding [ 23 ], for targeted versus untargeted funding [ 37 ], for ‘people grants’ versus project grants [ 36 , 38 ], for small grants versus large grants [ 10 ], and for competitive versus non-competitive research funding [ 39 ]. However, comparative evaluations of the impact of various funding models are scarce [ 10 , 23 , 38 ], even though approaches to measure the impact of health research are available [ 40 ]. An exception has been the recent comparisons of ‘people grants’ versus projects grants in the United States, which compared HHMI with NIH researchers and NIH Pioneer Awards with NIH project grants [ 36 , 41 – 43 ]. These comparisons have led the NIH to consider a broad shift toward ‘people grants’, demonstrating the value and potential impact of such evaluations [ 36 ]. Evaluations of this kind provide new insights when comparing funding models across funding organizations, but given the different contexts in which funders operate, comparing the impact of different models within one funding organization is perhaps particularly valuable and should become more common practice.
There is also a need for more debate about where the power to decide priorities for publicly funded health research should lie (with parliaments, ministries, funding agencies, or independent committees of experts). Such debate is needed because there are finite resources for investing in health research and thus priorities need to be set using fair and legitimate methods and using the best possible evidence [ 44 ]. In practice, public sector health research funding decisions are not only made on the basis of what research is needed, but are regularly influenced by other factors, such as political interests, advocacy and lobbying [ 2 ]. Thus, there is a need for transparency on who makes those decisions and to debate who should make them [ 2 , 13 , 45 – 47 ]. Analysis of funding organizations’ priority setting processes was not part of this study (see Limitations) but deserves to be a more frequent subject of research studies in the future.
Improving publicly available data on health research funding
Second, to enable evaluation and debates as noted above, it is necessary to have a map of the health research funding landscape: to know who the main funders of health research are, what they fund, and how they decide what gets funded [ 2 , 6 – 11 , 13 ]. Yet, this study shows that these data are often not available. Through our study, we did not find a list of all public or philanthropic health research funders worldwide that included their annual health research expenditures (Additional file 1 ). Therefore, we have now established such a list ourselves at www.healthresearchfunders.org . On this website, we provide access to the data collected for this article and to information on more than 200 other public and philanthropic funders of health research that we have added to this website since the mapping for this article was completed.
Besides the absence of a global listing of funding organizations, we found that data on organizations’ funding patterns and funding distribution mechanisms are often not available, and when they are, they are difficult to aggregate, owing to differences in funders’ data classification systems. Notably, we only collected these data for the 10 largest funding organizations of health research. The absence of such information, and the difficulties in aggregating the data across funders, are likely to be more prominent when smaller funders are also included. There is currently no consensus on a framework for producing descriptive data on funders’ funding patterns (both in terms of health areas and research types) nor on a framework for describing their funding distribution mechanisms [ 6 , 8 , 37 ]. In this article, we have proposed three frameworks for reporting data on health research funding: for reporting data on funding distribution mechanisms (Table 4 ), for reporting data on funding patterns in terms of health problems (the Global Burden of Disease classification [ 34 ]), and for reporting data on funding patterns in terms of research types (biomedical research, clinical research, epidemiological research or health systems research, as proposed by Frenk [ 35 ]). The adoption of standards for reporting funding data, including guidance on what data classification systems to use, by funding organizations, for example through collaborative initiatives such as the Heads of International Research Organizations, would substantially improve the quality and comparability of reported funding data [ 9 ].
Funding organizations are starting to support the goal of transparency and are increasingly recognizing the problems noted above and addressing them. At the 2014 World Health Summit in Berlin, several major funders of health research expressed interest to work together toward developing a common approach for mapping health research funding flows [ 12 ]. Another good example of a multi-funder collaboration to increase insight in health research investments is the World RePORT website [ 48 ]. On a national level, the United Kingdom has led the way in terms of harmonized reporting by showing it is feasible to collect comparable data on health research funding from all major public funding bodies and charities in a country [ 22 ]. Besides initiatives from funders themselves, there are also several promising initiatives from other parties to address the lack of data on global health research funding [ 1 , 16 , 49 – 51 ]. The recent decision to establish a Global Observatory on Health R&D at WHO in particular may help to improve transparency in this area [ 1 ].
Limitations
Finally, we note that the mapping conducted for this article has had several limitations. First, we have excluded funding organizations in the private for-profit sector (these are listed elsewhere [ 30 ]). Second, national systems for funding health research vary. In many countries, a large amount of health funding is dispersed directly from governments to universities or research institutes via block grants. We excluded these block grants and therefore the public funding organizations that we report on do not all contribute the same share of all health research that is publicly funded in a country. Third, we had to make several generalizations in order to be able to report data across funders that were diverse in their funding distribution mechanisms and reporting systems. For instance, what we have termed ‘targeted’ research funding, is a grey area that ranges from broad prioritized research areas to specific research topics or questions [ 52 ]. Also, funders reported on their expenditures on health research in various formats. Although we have kept track of these varying reporting formats, they decrease comparability across funders. Fourth, we would have liked to exclude overhead costs within project funding (not operational costs of the funder, which we did exclude where possible, but overhead costs of the research organization), to measure only the amount of funding that went to research, but this was not feasible because it was mostly not reported. Fifth, our proposed framework for reporting on funders’ funding distribution mechanisms (Table 4 ) lacks detail. It would have been interesting to also report on more detailed mechanisms, such as funders’ grants for businesses and PDPs/PPPs, but we did not include such analyses because of a lack of comparable data across funders. Sixth, funding organizations frequently make adaptations to their funding strategies, and therefore our findings should be viewed as a snapshot of funders’ expenditures, funding distribution mechanisms and funding patterns during the time of our data collection [ 53 ]. Seventh, in addition to reporting about funding organizations’ funding distribution mechanisms and patterns, we would have liked to report on funding organizations’ priority setting processes as part of this work (another important aspect of how funders decide what gets funded). However, we found that priority setting processes were generally not well-described and highly variable across funders, making it difficult to analyse and report our data. It deserves recommendation that such an analysis is conducted in the future, but the development of a framework for assessing priority setting processes at funders is needed first, potentially based on existing guidance for health research priority setting [ 44 ]. Lastly, and most importantly, our search strategy was limited in scope (see for more detail Additional file 1 ), was aimed only at finding the 10 largest funding organizations of health research in the world, and detailed data were only collected for those 10 organizations.
This study identified the 10 largest funding organizations of health research in the world and showed that these organizations together fund research for $37.1 billion, 40% of all public and philanthropic health research spending globally. It also mapped the funding patterns and funding distributions mechanisms of these funders and showed that there is considerable diversity between organizations in terms of what they fund and how they distribute those funds, highlighting the need for comparative evaluations of the impact of different funding distribution models. Moreover, because many of the data we tried to collect were not available, our study demonstrates that there is a need for increased transparency on who the largest funding organizations of health research are, what they fund, and how they decide what gets funded. As a first step in improving transparency in this area, we have proposed frameworks for reporting on funding patterns (in terms of health problems and research types) and for reporting on funding distribution mechanisms in this article and have established www.healthresearchfunders.org , where we list more than 250 public and philanthropic funders of health research and their annual health research expenditures. We will further expand and update this list of funding organizations in the future and welcome both suggestions and data from all who wish to help us make this database more accurate and more inclusive.
Røttingen J-A, Regmi S, Eide M, Young AJ, Viergever RF, Årdal C, et al. Mapping available health R&D data: what’s there, what’s missing and what role for a Global Observatory. Lancet. 2013;382:1286–307.
Article PubMed Google Scholar
Viergever RF. The mismatch between the health research and development (R&D) that is needed and the R&D that is undertaken: an overview of the problem, the causes, and solutions. Glob Health Action. 2013;6:22450.
PubMed Google Scholar
Power E. Impact of antibiotic restrictions: the pharmaceutical perspective. Clin Microbiol Infect. 2006;12 Suppl 5:25–34.
Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299–302.
Article CAS PubMed PubMed Central Google Scholar
Head MG, Fitchett JR, Cooke MK, Wurie FB, Atun R, Hayward AC, et al. Systematic analysis of funding awarded for antimicrobial resistance research to institutions in the UK, 1997–2010. J Antimicrob Chemother. 2014;69:548–54.
Article CAS PubMed Google Scholar
Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. Lancet. 2014;383:156–65.
Sampat BN, Buterbaugh K, Perl M. New evidence on the allocation of NIH funds across diseases. Milbank Q. 2013;91:163–85.
Article PubMed PubMed Central Google Scholar
Terry RF, Allen L, Gardner C, Guzman J, Moran M, Viergever RF. Mapping global health research investments, time for new thinking – A Babel Fish for research data. Health Res Policy Syst. 2012;10:28.
Viergever RF. Aid alignment for global health research: the role of HIROs. Health Res Policy Syst. 2011;9:12.
Couzin-Frankel J. Chasing the money. Science. 2014;344:24–5.
Track and trace. Nature. 2014;507:8.
World Health Summit. Workshop: global health research & development: mapping funding flows – working towards a common approach. Geneva: WHO; 2014.
Google Scholar
Gillum LA, Gouveia C, Dorsey ER, Pletcher M, Mathers CD, McCulloch CE, et al. NIH disease funding levels and burden of disease. PLoS One. 2011;6:e16837.
What has the Gates Foundation done for global health? Lancet. 2009;373:1577.
Matthews KR, Ho V. The grand impact of the Gates Foundation. Sixty billion dollars and one famous person can affect the spending and research focus of public agencies. EMBO Rep. 2008;9:409–12.
World Health Organization. WHO informal workshop – monitoring financial flows in support of health research & development. Geneva: WHO; 2013.
Young AJ, Terry RF, Røttingen J-A, Viergever RF. Global biomedical R&D expenditures. N Engl J Med. 2014;370:2451.
Chakma J, Sun GH, Steinberg JD, Sammut SM, Jagsi R. Asia’s ascent--global trends in biomedical R&D expenditures. N Engl J Med. 2014;370:3–6.
Sun GH, Steinberg JD, Jagsi R. The calculus of national medical research policy--the United States versus Asia. N Engl J Med. 2012;367:687–90.
Young AJ, Terry RF, Røttingen J-A, Viergever RF. Global trends in health research and development R&D expenditures – the challenge of making reliable estimates for international comparison. Health Res Policy Syst. 2015;13.
Myers ER, Alciati MH, Ahlport KN, Sung NS. Similarities and differences in philanthropic and federal support for medical research in the United States: an analysis of funding by nonprofits in 2006–2008. Acad Med. 2012;87:1574–81.
Reports & Downloads: Health Research Analysis Data. http://www.hrcsonline.net/pages/data . Accessed 13 January 2016.
Braun D. Structure and dynamics of health research and public funding: an international institutional comparison. Dordrecht: Kluwer Academic Publishers; 1994.
UK Clinical Research Collaboration. UK health research analysis 2009/2010. London: UKCRC; 2010.
Head MG, Fitchett JR, Cooke MK, Wurie FB, Hayward AC, Atun R. UK investments in global infectious disease research 1997–2010: a case study. Lancet Infect Dis. 2013;2013(13):55–64.
Article Google Scholar
Dorsey ER, de Roulet J, Thompson JP, Reminick JI, Thai A, White-Stellato Z, et al. Funding of US biomedical research, 2003–2008. JAMA. 2010;303:137–43.
Moran M, Guzman J, Henderson K, Liyanage R, Wu L, Chin E, et al. G-FINDER 2012 – neglected disease research & development: a five year review. Sydney: Policy Cures; 2012.
Frick M, Jiménez-Levi E. Tuberculosis Research and Development. Report on tuberculosis research funding trends, 2005–2012. New York: Treatment Action Group; 2013.
Head MG, Fitchett JR, Cooke GS, Foster GR, Atun R. Systematic analysis of funding awarded for viral hepatitis-related research to institutions in the United Kingdom. J Viral Hepat. 2015;22(3):230–7. doi: 10.1111/jvh.12300 .
The EU Industrial R&D Investment Scoreboard. http://iri.jrc.ec.europa.eu/scoreboard.html . Accessed 13 January 2016.
Saving Lives through Research: Annual Report and Accounts 2012/13. London: Cancer Research UK; 2013.
Research Facts 2012–13. American Heart Association; 2014. https://my.americanheart.org/idc/groups/ahamahpublic/@wcm/@sop/@rsch/documents/downloadable/ucm_317600.pdf . Accessed 13 January 2016.
International Monetary Fund (IMF) World Economic Outlook Database. https://www.imf.org/external/pubs/ft/weo/2014/01/weodata/index.aspx . Accessed 13 January 2016.
World Health Organization: Global Health Estimates. http://www.who.int/healthinfo/global_burden_disease/en/ . Accessed 13 January 2016.
Frenk J. The new public health. Annu Rev Public Health. 1993;14:469–90.
Kaiser J. Funding. NIH institute considers broad shift to “people” awards. Science. 2014;345:366–7.
Callahan D. Shaping biomedical research priorities: the case of the National Institutes of Health. Health Care Anal. 1999;7:115–29.
Ioannidis JPA. More time for research: fund people not projects. Nature. 2011;477:529–31.
Research funding needs overhaul. Science (80-) 2014, 345:122.
Banzi R, Moja L, Pistotti V, Facchini A, Liberati A. Conceptual frameworks and empirical approaches used to assess the impact of health research: an overview of reviews. Health Res Policy Syst. 2011;9:26.
Lal B, Wilson A, Jonas S, Lee E, Richards A, Peña V. An outcome evaluation of the national institutes of health (NIH) Director’s pioneer award (NDPA) program, FY 2004–2006. Washington: Ida Science & Technology Policy Institute; 2012.
Collins FS, Wilder EL, Zerhouni E. NIH roadmap/common fund at 10 years. Science. 2014;345:274–6.
Azoulay P, Graff Zivin JS, Manso G. Incentives and creativity: evidence from the academic life sciences. RAND J Econ. 2011;42:527–54.
Viergever RF, Olifson S, Ghaffar A, Terry RF. A checklist for health research priority setting: nine common themes of good practice. Health Res Policy Syst. 2010;8:36.
Hegde D, Sampat BN. Can private money buy public science? Disease group lobbying and federal funding for biomedical research. https://www8.gsb.columbia.edu/faculty-research/sites/faculty-research/files/canprivate.pdf . Accessed 13 January 2016.
Reardon S. Lobbying sways NIH grants. Nature. 2014;515:19.
Viergever RF, Hendriks TCC. Targeted public funding for health research in the Netherlands. Ned Tijdschr Geneeskd. 2014;159:A8174.
Collins F, Beaudet A, Draghia-Akli R, Gruss P, Savill J, Syrota A, et al. A database on global health research in Africa. Lancet Glob Heal. 2013;1:e64–5.
Rani M, Bekedam H, Buckley BS. Improving health research governance and management in the Western Pacific: a WHO expert consultation. J Evid Based Med. 2011;4:204–13.
Research classification in practice: Stamping or Understanding! http://www.uberresearch.com/uberresWP/wp-content/uploads/CASRAI-ReConnect-Research-Classification-2-UberResearch_March-2014.pdf . Accessed 13 January 2016.
Erawatch: Platform on Research and Innovation policies and systems. http://erawatch.jrc.ec.europa.eu/ . Accessed 15 August 2014.
The National Institute of Allergy and Infectious Diseases (NIAID): Choose Approach and Find FOAs. http://www.niaid.nih.gov/researchfunding/grant/strategy/pages/2choosefoa.aspx . Accessed 13 January 2016.
Wilkinson E. Wellcome Trust overhauls its funding framework. Lancet. 2014;384:1913.
G-FINDER public search tool. http://g-finder.policycures.org/gfinder_report/ . Accessed 13 January 2016.
Terry RF, van der Rijt T. Overview of research activities associated with the World Health Organization: results of a survey covering 2006/07. Health Res Policy Syst. 2010;8:25.
Download references
Acknowledgements
We would like to thank Alison Young, Koos van der Velden, Rob Terry, Noor Tromp, Leon Bijlmakers, Sanne van Kampen and Eric Budgell for reviewing drafts of this article.
Author information
Authors and affiliations.
Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, The Netherlands
Roderik F. Viergever
Department of Metamedica, VUmc, Amsterdam, The Netherlands
Thom C. C. Hendriks
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Roderik F. Viergever .
Additional information
Competing interests.
The authors declare that they have no competing interests. No specific funding was received for conducting this project.
Authors’ contributions
RV conceived the idea for the study, RV and TH developed the study methods, TH conducted most data collection and analysis, RV conducted additional data collection and analysis, and RV and TH wrote the article. Both authors read and approved the final manuscript.
Additional files
Additional file 1:.
More detailed description of methods. (DOCX 46 kb)
Additional file 2:
Sources for identification of health research funding organizations. (DOCX 45 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Viergever, R.F., Hendriks, T.C.C. The 10 largest public and philanthropic funders of health research in the world: what they fund and how they distribute their funds. Health Res Policy Sys 14 , 12 (2016). https://doi.org/10.1186/s12961-015-0074-z
Download citation
Received : 09 May 2015
Accepted : 18 December 2015
Published : 18 February 2016
DOI : https://doi.org/10.1186/s12961-015-0074-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Globalization
- Health funding
- Health policy
- Health research
- Priority setting
- Research and development (R&D)
- Research funding
- Research governance
- Research grants
- Research policy
Health Research Policy and Systems
ISSN: 1478-4505
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies (US) Committee on Measuring Economic and Other Returns on Federal Research Investments. Measuring the Impacts of Federal Investments in Research: A Workshop Summary. Washington (DC): National Academies Press (US); 2011.

Measuring the Impacts of Federal Investments in Research: A Workshop Summary.
- Hardcopy Version at National Academies Press
APPENDIX D THE IMPACT OF PUBLICLY FUNDED BIOMEDICAL AND HEALTH RESEARCH: A REVIEW 1
Bhaven N. Sampat
Department of Health Policy and Management
Columbia University
I. INTRODUCTION AND BACKGROUND
New biomedical technologies trigger a number of major challenges and opportunities in health policy. Among economists, there is widespread consensus that new technologies are the major drivers of increased healthcare costs but at the same time a major source of health and welfare improvements ( Murphy and Topel 2003 ). This has led to discussion about whether technological change in medicine is “worth it” ( Cutler and McClellan 2001 ). The impact of new technologies on the health care system has also been the subject of much debate among health policy scholars more generally ( Callahan 2009 ).
Public sector research agencies have an important role in the U.S. biomedical innovation system. In 2004, federal agencies funded roughly one-third of all U.S. biomedical R and D ( Moses et al. 2005 ). The National Institutes of Health (NIH) accounted for three-quarters of this amount. Private sector drug, biotechnology, and medical device companies provide the majority of U.S. biomedical R and D funding (about 58 percent). This private sector research is, in general, focused more downstream and tends to be closer to commercial application than NIH-funded research.
Donald Stokes (1997) observes that the public values science “not for what it is but what it is for.” A perennial question in U.S. science and technology policy is what benefits taxpayers obtain from publicly funded biomedical research. Recent concerns about the clinical and economic returns to NIH funding in the post-doubling era reflect this emphasis.
In this paper, we review the evidence on the effects of publicly funded biomedical research. Reflecting Stokes’s observation above, the review will focus on the health and economic effects of public research, rather than measures of scientific outcomes. Given the prominence of the NIH in funding this research, many of the published articles and research focus on this agency. The evidence examined includes quantitative analyses, and qualitative case studies, published by scholars from a range of fields. While we have made efforts to be broad, the references discussed should be viewed as representative rather than exhaustive. This review takes stock of the empirical methodologies employed and the types of data used; it also highlights common research and evaluation challenges, and emphasizes where existing evidence is more, or less, robust.
We proceed as follows. In Section II, below, we discuss a stylized model of how public research funding affects health, economic, and intermediate outcomes. As Kline and Rosenberg (1986) , Gelijns and Rosenberg (1994) , and others have emphasized, the research process cannot be reduced to a neat, linear model. While we recognize this fact (and highlight it in our literature review) the simple model is still useful in helping to organize our discussion of theory and data on the effects of publicly funded research. In Section III, we discuss the empirical evidence. In Section IV, we discuss common evaluation difficulties. In Section V, we conclude. The empirical approaches, data sources, and findings of many of the studies reviewed are also summarized in Tables D1 - D3 .
Public Funding and Health Outcomes: Summary of Selected Studies.
Public Funding and New Drugs, Devices: Summary of Selected Studies.
Public Funding and Private R and D, Patenting: Summary of Selected Studies.
II. PUBLIC SECTOR RESEARCH AND OUTCOMES: AN OVERVIEW
Figure D-1 is a simple model illustrating how the literature has conceptualized the health and economic effects of publicly funded biomedical research (and publicly funded research more generally):
Publicly Funded R and D and Outcomes, Logic Model. SOURCE: Sampat, 2011
The top arm of the model illustrates one important relationship: publicly funded R and D yields fundamental knowledge, which then improves the R and D efficiency of private sector firms, yielding new technologies (drugs and devices) that improve health outcomes. 2 This conceptualization has been the essential raison-d’ e tre for the public funding of science since Vannevar Bush’s celebrated postwar report, Science, The Endless Frontier . For example, Bush asserted in 1945 that “discovery of new therapeutic agents and methods usually results from basic studies in medicine and the underlying sciences” ( Bush 1945 ). It is also the essential mechanism in several important economic models of R and D (e.g. Nelson 1984). Importantly, this conceptualization generally views publicly funded research as “basic” research that is not oriented at particular goals, and thus yields benefits across fields. The influential “market failure” argument for public funding of basic research is that profit-maximizing, private-sector firms will tend to underinvest in this type of fundamental, curiosity driven research, since they cannot appropriate its benefits fully ( Nelson 1959 , Arrow 1962 ).
The channels through which publicly funded basic research might influence private sector innovation are diverse, including dissemination via publications, presentations and conferences, as well as through informal networks ( Cohen et al. 2002 ). Labor markets are another channel, since public agencies may also be important in training doctoral and post-doctoral students who move on to work for private sector firms ( Scherer 2000 ).
The second arrow illustrates another relationship. New instruments and techniques that are by-products of “basic” research can also improve private sector R and D ( Rosenberg 2000 ). Prominent examples of instruments and research tools emanating from academic research include the scanning electron microscope, the computer, and the Cohen-Boyer recombinant DNA technique.
Third, publicly-funded researchers sometimes develop prototypes for new products and processes. Some of these are indistinguishable from the informational outputs of basic research discussed above. For example, when academic researchers learned that specific prostaglandins can help reduce intraocular pressure this discovery immediately suggested a drug candidate based on those prostaglandins, though the candidate required significant additional testing and development. (This academic discovery later became the blockbuster glaucoma drug, Xalatan .) The public sector has also been important in developing prototypes ( Gelijns and Rosenberg 1995 ). Roughly since the passage of the Bayh-Dole Act, in 1980, publicly funded researchers have become more active in taking out patents on these inventions and prototypes for new products and processes, and licensing them to private firms ( Mowery et al. 2004 . Azoulay et al. 2007 ).
While much of the discussion of publicly funded biomedical research focuses on this more “basic” or fundamental research the public sector also funds more “applied” research and development. 3 For example, about one-third of the NIH budget is for clinical research, including patient oriented research, clinical trials, epidemiological and behavioral studies, as well as outcomes and health services research. Such research can be a useful input into the development of prototypes, and may also directly inform private sector R and D. Clinical research may also directly affect health behaviors. For example, knowledge from epidemiological research about cardiovascular health risk factors contributed to reductions in smoking and better diets ( Cutler and Kadiyala 2003 ). New applied knowledge can also influence physicians: for example, by changing their prescribing habits (e.g. “beta-blockers after heart attacks improve outcomes”) or routines (e.g. “this type of device works best in this type of patient”). Importantly, as various studies we review below will emphasize, negative results from clinical trials—showing that particular interventions do not work — can also be important for clinical practice and in shaping health behaviors.
While the discussion above assumes that new biomedical knowledge and technologies improve health outcomes, this is a topic of debate. The conventional wisdom is that while other factors (e.g. better diet, nutrition, and economic factors) were more important for health outcomes historically ( McKeown 1976 ), improvements in American health in the post-World War II era have been driven largely by new medical knowledge and technologies ( Cutler, Deaton, and Lleras-Muney 2006 ). The contribution of publicly funded research to these developments is an open empirical question, discussed below.
At the same time, some scholars suggest that we may have entered an era of diminishing returns, where new technologies are yielding increasingly less value ( Callahan 2009 ; Deyo and Patrick 2004). The effect of new biomedical technologies on healthcare costs is a related concern. There is general agreement among health economists that new medical technologies are the single biggest contributor to the increase in long-run health costs, accounting for roughly half of cost growth (Newhouse 1992). Rising health costs strain the budgets of public and private insurers as well as employers, and may also contribute to generate health inequalities. The dynamic that exists between new medical technologies and health costs in the U.S. may reflect a “technological imperative,” which creates strong incentives for the healthcare system to adopt new technologies once they exist (Fuchs 1995; Cutler 1995 ). It may also reflect positive feedbacks between demand for insurance and incentives for innovation ( Weisbrod 1991 ).
Concern about the effects of technology on health costs has fueled empirical work on whether technological change in medicine is “worth it.” Long ago, Mushkin (1979) noted (though did not share) “widespread doubt about the worth of biomedical research given the cost impacts.”
A large literature in health economics suggests that new biomedical technologies are indeed, in the aggregate, worth it. Cutler (1995) and others suggest that, given the high value of improved health (current estimates suggest the value of one additional life year is $100,000 or more), even very costly medical technologies pass the cost-benefit test. 4 Nordhaus (2003) estimates that the value of improvements in health over the past half century are equal in the magnitude to measured improvements in all non-health sectors combined. Others ( Callahan 2009 ) view these health cost increases as unaffordable, even if they deliver significant value, and therefore ultimately unsustainable.
At the same time, not all medical technologies necessarily increase costs. As Cutler (1995) and Weisbrod (1991) indicate, technologies that make a disease treatable but do not cure it - moving from non-treatment to “halfway” technology in Lewis Thomas’s characterization-are likely to increase costs. The iron-lung to treat polio is an example of this. However, technologies that make possible prevention or cure (“high technology”) can be cost-reducing, especially relative to halfway technologies. Thus the polio vaccine was much cheaper than the iron lung. Consistent with this, Lichtenberg (2001) shows that while new drugs are more expensive than old drugs, they reduce other health expenditures (e.g. hospitalizations). Overall, he argues, they result in net decreases in health costs (and improve health outcomes). 5
As Weisbrod (1991) notes, “The aggregate effect of technological change on health care costs will depend on the relative degree to which halfway technologies are replacing lower, less costly technologies, or are being replaced by new, higher technologies. ” 6 One way to think about the effects of public sector spending on costs would be to assess the propensity of publicly funded research to generate (or facilitate the creation of) these different types of technologies. However, since the effects of these new technologies are mediated by various facets of the health care and delivery system, it may be difficult conceptually (and empirically) to isolate and measure the effects of public sector spending on overall health costs ( Cutler 1995 ). 7
II. THE EFFECT OF PUBLICLY FUNDED RESEARCH: A REVIEW OF THE EVIDENCE
Measuring the health returns to publicly funded medical research has been a topic of interest to policymakers for decades. In an early influential study, Comroe and Dripps (1976) consider what types of research (basic or clinical) are more important to the advance of clinical practice and health. The authors rely on interviews and expert opinion to determine the top ten clinical advances in the cardiovascular and pulmonary arena, and identified 529 key articles associated with these advances. They coded each of the key articles into six categories: (1) Basic research unrelated to clinical problems; (2) Basic research related to clinical problems (what Stokes later termed “use-oriented” basic research); (3) Research not aimed at understanding of basic biological mechanisms; (4) Reviews or syntheses; (5) Development of techniques or apparatuses for research; and (6) Development of techniques or apparatuses for clinical use. The authors find that 40 percent of the articles were in category 1, and 62 percent in categories 1 or 2. Based on this, the authors assert “a generous portion of the nation’s biomedical research dollars should be used to identify and then to provide long-term support for creative scientists whose main goal is to learn how living organisms function, without regard to the immediate relation of their research to specific human diseases.” Comroe and Dripps also note “that basic research, as we have defined it, pays off in terms of key discoveries almost twice as handsomely as other types of research and development combined” (1976).
A more recent set of studies examines the effects of publicly funded research on health outcomes. Operationalizing the concept of “health” is notoriously difficult. Common measures employed to account for both the morbidity and mortality effects of disease include quality adjusted life years (QALYs) and disability adjusted life years (DALYs) ( Gold et al, 2002 ). However, it is difficult to get longitudinal information on these measures by disease. As a result, most of the analyses of the effects of public funding on health examine more blunt outcomes, including the number of deaths and mortality rates for particular diseases.
Numerous prominent academic studies ( Weisbrod 1983 , Mushkin 1979 ) aim to examine the health effects of biomedical research, and the economic value of this impact, in a cost-benefit framework. One important recent study in this tradition, Cutler and Kadiyala (2003) , focuses on cardiovascular disease—the disease area where there has been the strongest improvement in health outcomes over the past sixty years. Since 1950 mortality from cardiovascular disease decreased by two- thirds, as Figure D-2 (reprinted from their paper) shows:
Mortality by cause of death 1950–1994. SOURCE: Cutler and Kadiyala 2003
Cutler and Kadiyala, through a detailed review of the causes of this advance (relying on epidemiological and clinical data, medical textbooks, and other sources), estimate that roughly one third of this cardiovascular improvement is due to high-tech treatments, one third to low tech treatments, and one third to behavioral changes. Assuming one additional life year gained is valued at $100,000, the authors compute a rate of return of 4-to-1 for investments in treatments and 30-to-1 for investments in behavioral changes. These investments include costs borne by consumers and insurers, and estimates of public sector R and D for cardiovascular disease.
Based on these figures, the authors argue that the rate of return to public funding is high, though they don’t directly trace public funding to changes in outcomes in their quantitative analyses. Interestingly, in their qualitative account, the major public sector research activities highlighted have an “applied” orientation, including the NIH’s role in sponsoring large epidemiological trials and holding consensus conferences. This may reflect a traceability and attribution problem, which is common to the evaluation of fundamental research: It is difficult to directly link improvements in outcome indicators to public sector investments in basic research, even in a study as detailed as this one.
A paper by Heidenreich and McClellan (2003) is similarly ambitious, looking at sources of advance in the treatment of heart attacks. The authors focus on this treatment area, not only because of the large improvements, but also because it is a “best case” for attributing health outcomes to particular biomedical investments. Specifically, these authors go further than Cutler and Kadiyala by attempting to link changes in clinical practice to changes in specific R and D inputs. The authors focus here on clinical trials, not basic research. This is not because they believe that basic research is unimportant, “but because it is much easier to identify connections between these applied studies and changes in medical care and health.”
Based on detailed analyses of MEDLINE-listed trials and health outcomes, the authors argue that medical treatments studied in these trials account for the bulk of improvement in AMI outcomes. The authors associate changes in clinical practice and outcomes to research results reported in trials through analysis of timing of events, and detailed clinical knowledge of how the trial results, clinical practices, and health outcomes relate.
One interesting result from this paper is that clinical practice often “leads” formal trials, challenging the “linear” model embodied in Figure D-1 (above). The authors also emphasize that an important role for trials is negative: telling clinicians what doesn’t work, and stopping the diffusion of ineffective technologies. While the sample they examine represents a mix of publicly funded and privately funded trials, the authors do emphasize a particularly important role for the public sector in funding trials on drugs off patent, where private firms have fewer incentives to do so.
Philipson and Jena’s (2005) study of HIV-AIDS drugs is another paper that examines the value of increases in health from new medical technologies. Though this study does not explicitly focus on the role of the public sector, it estimates that HIV-AIDS drugs introduced in the 1990s generated a social value of $1.4 trillion, based on the value of the increments to life expectancy created from these drugs (here again, using the estimate of $100,000 per life year). This study is relevant because of the important role of public sector research in the development of HIV‐AIDS drugs, which is observed in several of the empirical studies discussed below.
A recent paper by Lakdawalla et al (2011) employs a similar approach to assess the benefits from cancer research. The authors find these benefits to be large, estimating the social value of improvements from improvements in life expectancy during the 1988–2000 period to be nearly $2 trillion. The authors note that this compares to investments of about $80 billion dollars in total funding for the National Cancer Institute between 1971 and 2000. As with the HIV studies discussed above, the authors do not calculate a rate-of-return on publicly funded research explicitly, but do argue that the social benefits from cancer research in general far exceed research investments and treatment costs.
A large share of the benefits in the cancer arena, according to this work, results from better treatments. Lichtenberg (2004) also suggests that new drug development has been extremely important in progress against cancer. 8 Public sector research may have been important to the development of these drugs: various studies ( Stevens et al. 2011 , Chabner and Shoemaker 1989) suggest an important role for the public sector in cancer drug development. 9
Each of the studies discussed so far focuses on particular disease areas. In a more “macro” approach Manton (2009) and colleagues relate mortality rates in four disease areas to lagged NIH funding by the relevant Institute, over the period 1950–2004. They find that for two of the four diseases (heart disease, stroke) there is a strong negative correlation, but find weaker evidence for cancer and diabetes. Several issues arise here that will re-emerge in other quantitative analyses discussed below. First, linking funds to disease areas is difficult. As with other studies we will consider below, the authors here rely on the disease foci of Institutes within the NIH. More importantly, the counterfactual is hard to prove: It is difficult to make the case that the relationships estimated are causal, since Institute-specific funding is not exogenous. In particular, diseases where there is highest expectation of progress (even absent funding) may be more likely to get funds. Finally, competing risks also complicate interpretation of health outcomes. For example, part of the reason cancer mortality has increased rather than decreased over the period studied is that people no longer die of heart attacks, due to advances in the cardiovascular arena.
Private Sector R and D
Another set of studies relates publicly funded research to private sector R and D and productivity. These include econometric analyses relating public sector and private sector funding, surveys of firm R and D managers, and studies examining the geographic dimension of spillovers from public sector researchers.
Several papers relate NIH funding by disease area to later private sector funding. One motivation in these studies is to assess if public and private sector R and D are substitutes or complements, an issue of perennial interest in science and technology policy ( David, Hall, and Toole 2000 ). The econometric analyses generally find a positive association between public sector and private sector funding. Toole (2007) uses data from the NIH’S Computerized Retrieval of Information on Scientific Projects (CRISP) database, covering NIH basic and clinical research funding across seven therapeutic classes (between 1972 and 1996), and data from the Pharmaceutical Manufacturers of America (PhRMA) on private sector R and D in these same areas (between 1980 and 1999) to examine the relationships between the two. This study finds a 1 percent increase in basic research funding associated with a 1.7 percent increase in private sector funding, though the elasticity for clinical research is much smaller (.4 percent). In a similar analysis, Ward and Dranove (1995) , using PhRMA data on R and D spending and NIH data on funding by Institute (similar to that used in the Manton et al 2009 study discussed above) find that a 1 percent increase in NIH research support in a disease area is associated with a .76 percent increase in private sector R and D within that same disease area over the next seven years.
Surveys of firm R and D managers have also been used to gauge how public sector research affects private sector R and D. Cohen, Nelson, and Walsh (2002) report on the 1994 Carnegie Mellon Survey of Industrial R and D managers, which examined (among other issues) the roles of the public sector in industrial R and D, and channels through which public research affects industrial R and D. This survey is particularly interesting since it has data on both the drug and device sectors, and allows for comparison of these sectors to others. The authors find that the pharmaceutical industry is an outlier in its reliance on public sector R and D. In the pharmaceutical industry, according to respondents, public research was the most important source of new project ideas and contributor to project completion. By contrast, in the medical instruments industry R and D projects less frequently rely on public research than other industries. There are also some differences in the fields of science relied upon across these different industries. Thus the top three fields of science important to R and D projects in the pharmaceutical industry are medicine, biology, and chemistry. In medical instruments sector, the top three fields are medicine, materials science, and biology. Although much of the literature on the effects of public sector funding tend to focus on the NIH, the bulk of funding for materials science R and D comes from other agencies (including the National Science Foundation, Department of Energy, and the Department of Defense).
Another set of studies, examining how interactions between public and private sector scientists affects the productivity of private sector R and D, generally finds a strong relationship between the two. Cockburn and Henderson (1996) examine how private sector co-authorship with public sector scientists affects firm level R and D and productivity. The authors bring together several novel datasets, including MEDLINE data on firm publication activity and USPTO data on firm patenting activity. Using panel regression models (with firm fixed effects to control for time-invariant firm characteristics), they find a positive and statistically significant association between their productivity measure (based on important patents per R and D dollar) and collaboration with public sector scientists.
Research by Zucker, Darby, and Brewer (1998) examines the importance of academic science in the creation of new biotechnology firms in the 1980s. In this work, the authors relate new biotechnology firm formations by area to the number of academic “star scientists” (as measured by publications and other measures of scientific productivity) working in that area. The authors find that the presence of academic stars and their collaborators— intellectual capital”—within a geographic area has a statistically significant and positive relationship with the number of new biotechnology enterprises later formed in that area. This research suggests that public sector science has an important, though geographically mediated, effect on private sector research.
The question of whether spillovers from public research to firms are geographically mediated has also been examined through studies using patent citation data ( Jaffe et al. 1993 ). When patents are granted they include citations to prior art: earlier publications and patents that were deemed (by either the applicant or the patent examiner) as relevant to an invention. Economists and others have interpreted patent citations as evidence of knowledge flows or spillovers: thus if a firm patent cites a public sector publication or patent, this is considered evidence that the firm benefited from public funding. While there is some skepticism about this measure, given the prominence of patent examiners in generating citations ( Alcacer et al. 2009 ; Cohen and Roach 2010 ), it remains commonly employed. Moreover, as it turns out, examiner-added citation are less common within the biomedical arena ( Sampat 2010 ) and for citations to scientific publications ( Lemley and Sampat 2011 ) suggesting that citations in biomedical patents to scientific publications may be less subject to the concerns cited above.
Azoulay, Graff Zivin, Sampat (2011) collected data on 10,450 elite life science researchers (most of them publicly funded), historical information on productivity, employment locations of each scientist, MEDLINE data on their publications, ISI data on citations to their publications, and USPTO data on their patents and citations to their patents and publications. The authors assess the effects of geography on spillovers by examining how citation patterns change after the scientists move. Overall, they find some evidence that geography matters for spillovers, though weaker than in previous analyses. They also find the results on geography are sensitive to whether spillovers are measured through paper-to-paper citations, patent-to-patent citations, or patent-to‐paper citations.
Private Sector Innovation
Numerous studies also consider the public sector role in the development of marketed innovations. Survey work by Mansfield (1998) examines the importance of academic research for industrial innovation for firms across a range fields. In this work, as in the Carnegie Mellon Survey discussed above, the biomedical industries are outliers. The share of products developed over the late 1980s and early 1990s that could not have been developed (without substantial delay) absent recent academic research is nearly twice as high in drugs and medical products than in other industries.
Various recent studies examine the roles of the public sector in drug development using patent and “bibliometric” data. In addition to providing an indicator of returns to public R and D, this work may also be relevant to current policy proposals that aim to exploit public sector ownership of drugs to help reduce downstream drug prices and expand access ( Sampat and Lichtenberg 2011 ).
Sampat (2007) uses data on all drugs approved by the Food and Drug Administration (FDA) between 1988 and 2005 (and listed on the FDA’s Orange Book ), and USPTO data on patents associated with these drugs, to examine the share of drugs on which academic institutions (including public sector laboratories) own patents. Overall, a small number of new molecular entities (NMEs), about 10 percent, have academic patents. However, this share is larger for new molecular entities that received priority review (arguably the most innovative new drugs), where about 1-in-5 drugs have academic ownership. He also finds that public sector ownership of drugs is more pronounced for HIV‐AIDS drugs than for other drug classes.
Stevens et al. (2011) expand on this research to include vaccines and biologicals (not always listed on the Orange Book), and construct measures based not only on publicly available patent data but also propriety data on drug licenses. They find 153 FDA-approved drugs were discovered by the public sector over the past 40 years (102 NMEs, 36 biologics, and 15 vaccines.) The authors show that about 13 percent of NMEs (and 21 percent of priority NMEs) were licensed from public sector institutions, consistent with the numbers reported in Sampat (2007). Strikingly, the authors also show that virtually all the important vaccines introduced over the past quarter century came from the public sector. The authors also show broad correlations between NIH Institute budgets and the therapeutic classes where there are numerous public-sector based drugs, similar in spirit to econometric analyses we will review below.
Kneller (2010) takes a different approach, relying not on patent assignment records but instead on information related to the inventors’ places of employment, and applies his analysis to 252 drugs approved by the FDA between 1998 and 2007. Using these measures, Kneller finds a larger public sector influence than the previous studies. Overall, about a quarter of drugs are from university inventors, and a third of priority review drugs are from academic inventors.
The Sampat, Stevens et al, and Kneller studies rely on direct academic involvement in developing the molecules (resulting in academic ownership of the key patents or academic inventors listed on those patents). However, as discussed in Section II, in addition to the development prototypes, the public sector can facilitate or enhance industrial innovation in other ways as well. Thus Keyhani et al (2005) , using data from the Federal Register, government clinical trials databases, and documents from the FDA, finds the government was active in supporting clinical trials for nearly 7 percent of a sample of drugs approved between 1992 and 2002. Here again, the government role was more pronounced for HIV-AIDS drugs than for others.
Sampat and Lichtenberg (2011) distinguish between the direct effects of public sector research on drug development, where academic institutions are involved in discovering the molecule, and the indirect effects, where other knowledge spillovers from academic work increase private sector productivity. The authors measure the direct effect of public sector funding using information on “government interest” statements in Orange Book listed patents. And they use citations in Orange Book listed patents to academic patents or academic publications as a measure of this indirect effect. Consistent with the various studies cited above, this study suggests the direct effect is small overall: about 9 percent of drugs, and about 17 percent of priority review drugs, have public sector owned patents. However the indirect effect is much larger: about 48 percent of drugs have patents that cite public sector patents and publications. Among priority drugs, this indirect influence rises to nearly two-thirds. This finding is broadly consistent with the qualitative results from Cockburn and Henderson’s (1996) study of fifteen drugs, which shows the public sector made key enabling discovery for the majority (11 of the 15), but was involved in synthesis of the compound for only 2 of the 15.
The studies discussed above are accounting exercises. Others also have attempted to relate variation in funding by disease area to drug development patterns, econometrically. Dorsey et al. (2009) relate NIH funding by therapeutic area to later drug approvals across nine disease areas between 1995 and 2000. The authors allocate funding to specific diseases based on funding Institute using information in Congressional budget requests for those institutes. They find that despite a sharp rise in NIH funding over this time period, drug approvals remained flat overall. And their cross-therapeutic area analyses show little correlation between NIH funding and subsequent drug approvals.
Blume-Kohut (2009) also explores these issues, using panel regression models. She constructs data on NIH funding by disease area between 1975 and 2004 from the agency’s CRISP and RePORTER databases, based on parsing of abstracts and keywords of grants for disease keywords. She also examines information on drugs in development by class using data from a private data vendor, PharmaProjects. Her results show little evidence of responsiveness between the number of drugs in Phase III trials (late stage) and NIH funding, but evidence of a positive relationship for the number of drugs in earlier stage Phase I trials. The author notes these results may suggest that factors other than NIH funding (or the state of knowledge) may be important for Phase III trials, including commercial considerations such as the size of the market. In a similar approach, using a different outcome measure, Ward and Dranove (1995) relate MEDLINE publications tagged as “drug” articles to NIH R and D funding by disease area, here again categorized based on funding institute. They find a strong relationship between the two.
Most of the studies we have discussed thus far, examining public sector research and product development, focus on drugs and involve quantitative analysis. By contrast, Morlacchi and Nelson (2011) examine the sources of innovation in the development of the left ventricular assist device (LVAD), a medical device used for patients with end-stage heart failure. While the device originally was developed as a “bridge” solution until a heart became available for transplant, it is increasingly used as destination therapy, as a substitute for a heart transplant. Morlacchi and Nelson draw on interviews, primary and secondary articles, and patents to develop a longitudinal history of the development of the LVAD. They consider, among other questions, the importance of public sector funding in this development. Echoing some of the themes in Heidenreich and McClellan’s study of heart attack treatment, they find that in this field application led scientific understanding. The development of the device occurred even as basic understanding of heart failure remained weak, once again challenging the linear model of innovation portrayed in Figure D-1 . They also find that the applied and diffusion oriented activities of public sector funders were important in the development of this device, including the NIH’s sponsorship of conferences and centers to spread best-practice, funding of trials and development of important component technologies, and contracts to spur firm formation.
Health Costs
Despite longstanding concerns about the effects of new biomedical technologies on healthcare costs, and speculation that public sector research may be implicated in spurring this cost spiral, there has been surprisingly little empirical research on this topic. For example, there is a paucity of academic work relating funding patterns by disease area to subsequent cost growth, analogous to the work relating funding to private sector R and D, drug development, and health outcomes discussed above.
In 1993, the NIH prepared studies on the cost savings from a non‐random sample of 34 health technologies resulting from NIH support, demonstrating substantial cost savings ( NIH 1993 ). This study examined NIH funding for new technologies, as well as cost savings that accrued to patients, based on conservative assumptions on reductions in disease attributable to those same technologies. An NIH summary (NIH 2005) of this work notes that,“[t]aken together, the 34 technologies were estimated to reduce health care costs by about $8.3 billion to $12.0 billion annually.” As with several studies discussed earlier, difficulty in tracing the effects of “basic” research to particular technologies may complicate such calculations. Moreover, as the agency’s summary emphasizes “because the 34 new health care technologies studies were not chosen to be representative of all health advances resulting from NIH support, the results of these case studies cannot be generalized.”
While there has been little work, beyond this NIH study, on the effects of public sector funding on the direct costs of disease (i.e. health expenditures), the various studies discussed above that address the value of new biomedical technologies, can be interpreted as evidence that public sector funding reduces the total cost of disease, to the extent that the estimated improvements in health are viewed as reductions in the social costs associated with disease.
III. MEASUREMENT AND EVALUATION ISSUES
The diverse set of studies reviewed here illustrates a number of common measurement and evaluation issues that complicate efforts to estimate the health and economic effects of publicly funded biomedical research. Here, we will highlight several that stand out.
Several of the studies reviewed relate public sector funding by disease area to outputs. All of these focus on the NIH, since for other agencies publicly available data on funding by disease area is not readily available. Even for the studies focused on the NIH, however, there are measurement issues. While many studies construct funding stocks based on which Institutes fund the research, Institutes fund numerous diseases, introducing considerable noise into these measures.
The NIH’s CRISP database includes disease keywords, which can also be used to construct disease specific funding, but these are not collected in a standard way across the NIH ( Sampat 2011 ). In 2008, the NIH launched the “Research, Condition, and Disease Categorization” (RCDC) database, which uses standard methodologies to classify funds by area. Whereas, previously, each NIH Institute had linked its grants to diseases in an ad hoc and non-standard way, the RCDC employs standard category definitions to classify grants, developed with input from disease groups, the scientific community, and outside consulting groups. Before the RCDC, the NIH had provided disease-specific funding figures tentatively and with many caveats. Today, with the existence of the RCDC database, the agency has exhibited a more firm commitment to its own data sources and tracking. The NIH website thus affirms: “RCDC provides consistent and transparent information to the public about NIH-funded research. For the first time, a complete list of all NIH-funded projects related to each category is available.” This database may prove a boon for future researchers. However, its time frame and scope (covering only diseases and conditions “of historical interest to Congress”) may limit the types of analyses that can be conducted using these data.
A more fundamental issue is difficulty in categorizing “basic” research in these studies. Thus in the CRISP funding database, 49% of grants awarded in 1996 (accounting for 46% of NIH allocations) listed no disease terms, and only about 45% of grants map to a disease category in the RCDC ( Sampat 2011 ). It is difficult to incorporate these grants into disease level associations of funding and outputs. Basic research is also difficult to trace to outcomes even in a case study context, given lags and diffuse channels of impact. Thus it is not surprising that several of the evaluation studies discussed above (including the study of heart attack treatment, and the studies of NIH research and costs) focus on the effects of applied research.
The bibliometric approaches discussed above, linking grants to publications to citations to patents to drugs may overcome these traceability challenges, relying on paper trails between research and outcomes, and avoiding the need to associate public sector funding with particular diseases. However, the validity of these analyses rest on a number of assumptions, e.g. the extent to which patent-paper citations reflect real knowledge flows from public sector research.
Thus, measurement of inputs and intermediate steps is difficult. Measuring outcomes is conceptually easier, at least relative to evaluation of research outputs in non-biomedical contexts. Though the right output measures (e.g. morbidity or mortality, direct or indirect costs) or desiderata (should the NIH be mainly focused on advancing health? science? competitiveness? something else?) are the subject of debate, there is a wealth of data available to examine changes in health-related outcomes. Similarly, the research community has exploited numerous useful measures of relevant economic outcomes (e.g. patents, drug development, publications), again more readily available in the biomedical context than other arenas.
Causal evaluation of the effects of publicly funded research on these outcomes is difficult however, in this context and in S and T policy more generally. Simply put, funding choices are not random, making it difficult to attribute observed changes in outcomes to specific policies. As just one example, if public sector funding targets disease areas with high scientific opportunity, it is difficult to untangle whether subsequent improvements in health (or changes in private sector R and D, or drug development) reflect the effects of the funding or of the scientific opportunity. Several of the studies discussed attempt to address this problem econometrically, including through panel regression models with disease fixed effects, to absorb the effects of disease-specific characteristics that do not change over time. Going forward, quasi-experimental techniques may also prove useful. For example, it may be possible to exploit random shocks to funding in particular areas that are unrelated to scientific opportunity and disease burden could (e.g. those introduced through political influence on the allocation process, or changes in agencies’ funding rules) to assess the effects of public research.
There is also a need for more qualitative work. A number of the case studies surveyed above relied on detailed knowledge of the institutions at play, in depth clinical knowledge, and information on the timing of relevant events, to make credible arguments that the relationships they observed were causal. These too represent promising research approaches going forward.
IV. CONCLUSIONS
The measurement evaluation challenges highlighted above are endemic to science and technology policy in general (Jaffe 1998). A main output of science and technology policy is knowledge, which is difficult to measure and link to downstream outcomes. This exacerbates traditional difficulties with attributing causal effects to policy interventions, common to evaluation in most public policy domains.
Notwithstanding these challenges, at least on several issues various studies point in the same direction. First, there is consistent evidence across on the importance of public sector biomedical R and D for the efficiency of private sector R and D. The evidence is compelling since it is based on a range of studies using different techniques and samples, including surveys, case studies, and econometric analyses.
Second, the accounting studies on sources of innovation in drugs suggest that the public sector was directly involved in the development of a small share of drugs overall, but that the public sector role is more pronounced for more “important” drugs, and that the indirect effect of public sector research on drug development is larger than the direct effect. On the other hand, the studies that relate patterns of funding by disease area to drug development show less consistent results.
Third, a number of the studies suggest the importance of the applied and clinical public research activities on product development, patient behaviors, and health outcomes. This is striking, since much of the discussion about publicly funded biomedical research focuses on (and most of the funding is for) “basic” research. Whether the importance of applied activities reflects that their effects are easier to measure and trace, or that they are really very important, is an open empirical question. 10
Overall, there is strong evidence that new biomedical technologies have created significant value, as measured through the economic value of health improvements. Some scholars believe that even if public sector research was responsible for only a small share of this gain, it delivers high returns on investment ( Murphy and Topel 2003 ). 11
More work is needed directly examining the role of the public sector per se, and especially public sector basic research, in affecting these health outcomes. Similarly, very little is known about the effects of public sector research on health expenditures. Detailed longitudinal case studies of trends in public and private sector research activity, technology utilization, health outcomes, and health expenditures across a number of disease areas would be useful for promoting understanding on each of these issues. To the extent possible, it would be useful for these studies to employ common methods and measures, and to examine both disease areas where there has been considerable advance, and those where there has been less progress.
Finally, the bulk of the academic work in this area focuses on the NIH and pharmaceuticals. Much more research is needed on the effects of other funding agencies, and on the effects of public funding on the device sector.
- Alcacer J, Gittleman M, Sampat BN. Applicant and Examiner Citations in U.S. Patents: An Overview and Analysis. Research Policy. 2009; 38 (2):415–427.
- Arrow K. Economic Welfare and the Allocation of Resources for Invention. In: Nelson Richard., editor. The Rate and Direction of Inventive Activity. Princeton, NJ: Princeton University Press; 1962.
- Azoulay P, Michigan R, Sampat BN. The Anatomy of Medical School Patenting. The New England Journal of Medicine. 2007; 20 (357):2049–2056. [ PubMed : 18003961 ]
- Azoulay P, Zivin JG, Sampat BN. The Diffusion of Scientific Knowledge across Time and Space: Evidence from Professional Transitions for the Superstars of Medicine. NBER Working Paper 16683. 2011
- Ballar J, Gornick H. Cancer Undefeated. New England Journal of Medicine. 1997; 336 :1569–1574. [ PubMed : 9164814 ]
- Blume-Kohout ME. Drug Development and Public Research Funding: Evidence of Lagged Effects. Waterloo, ON, Canada: University of Waterloo; 2009. pp. 1–35.
- Bush V. Washington, DC: United States Government Printing Office; Science, The Endless Frontier. 1945
- Callahan D. Princeton: Princeton University Press; Taming the Beloved Beast: How Medical Technology Costs Are Destroying Our Health Care System. 2009
- Chabner BA, Shoemaker D. Drug Development for Cancer: Implications for Chemical Modifiers. International Journal of Radiation Oncology Biology Physics. 1988; 16 :907–909. [ PubMed : 2703396 ]
- Chandra A, Skinner J. Technology Growth and Expenditure Growth in Health Care. NBER Working Paper 16953. 2011 [ PubMed : 21957511 ]
- Cockburn IHR. Public-Private Interaction in Pharmaceutical Research. Proceedings National Academy of Science USA. 1996; 93 (23):12725–12730. [ PMC free article : PMC34128 ] [ PubMed : 8917485 ]
- Cohen WM, Nelson R, Walsh J. Links and Impacts: The Influence of Public Research on Industrial R and D. Management Science. 2002; 48 (1):1–23.
- Cohen W, Roach M. Patent Citations As Indicators of Knowledge Flows From Public Research. Working Paper. 2010 [ PMC free article : PMC3901515 ] [ PubMed : 24470690 ]
- Comroe J, Dripps RD. Scientific Basis for the Support of Biomedical Science. Science. 1976; 192 (4235):105–111. [ PubMed : 769161 ]
- Cutler D. Technology, Health Costs, and the NIH. Paper prepared for the National Institutes of Health Economic Roundtable on Biomedical Research. 1995.
- Cutler DM. Are We Finally Winning the War on Cancer? Journal of Economic Perspectives. 2008; 22 (4):3–26. [ PubMed : 19768842 ]
- Cutler D, McClellan M. Is Technological Change in Medicine Worth It? Heath Affairs. 2001; 20 (5):11–29. [ PubMed : 11558696 ]
- Cutler D, Kadiyala S. The Returns to Biomedical Research: Treatment and Behavioral Effects. In: Murphy Kevin, Topel Robert., editors. Measuring the Gains from Medical Research: An Economic Approach. Chicago: University of Chicago Press; 2003. pp. 110–162.
- Cutler D, Deaton A, Lleras-Muney A. The Determinants of Mortality. The Journal of Economic Perspectives. 2006; 20 (3)
- David PA, Hall BA, Toole AA. Is Public R and D a Complement or Substitute for Private R and D? A Review of the Econometric Evidence. Research Policy. 2000; 29 :497–529.
- Dorsey ER, Thompson JP, Carrasco M, de Roulet J, Vitticore P, Nicholson S, Johnston SC, Holloway RG, Moses H III. Financing of U.S. Biomedical Research and New Drug Approvals across Therapeutic Areas. PloS One. 2009; 4 (9):e7015. [ PMC free article : PMC2735780 ] [ PubMed : 19750225 ]
- Dorsey ER, Vitticore P, De Roulet J, Thompson JP, Carrasco M, Johnston SC, Holloway RG, Moses H III. Financial Anatomy of Neuroscience Research. Annals of Neurology. 2006; 60 (6):652–659. [ PubMed : 17192926 ]
- Fuchs V. Cambridge, MA: Harvard University Press; The Health Economy. 1986
- Gelijns A, Rosenberg N. The Dynamics of Technological Change in Medicine. Health Affairs. 1994; 13 (3):28–46. [ PubMed : 7927160 ]
- Gelijns A, Rosenberg N. The Changing Nature of Medical Technology Development. In: Rosenberg N, Gelijsn A, Dawkins H, editors. Sources of Medical Technology: Universities and Industry. Washington National Academies Press; 1995. [ PubMed : 25121227 ]
- Gold M, Stevenson D, Fryback D. HALYS and QALYS and DALYS, OH MY: Similarities and Differences in Summary Measures of Population Health. Annual Review of Public Health. 2002; 23 :115–134. [ PubMed : 11910057 ]
- Heidenreich P, McClellan M. Biomedical Research and Then Some: The Causes of Technological Change in Heart Attack Treatment. In: Murphy Kevin, Topel Robert., editors. Measuring the Gains from Medical Research: An Economic Approach. Chicago: University of Chicago Press; 2003. pp. 163–205.
- Jaffe A, Trajtenberg M, Henderson R. Geographic Localization of Spillovers as Evidenced By Patent Citations. The Quarterly Journal of Economics. 1993; 108 (3):577–598.
- Keyhani S, Diener-West M, Powe N. Do Drug Prices Reflect Development Time and Government Investment? Medical Care. 2005; 43 (8):753–762. [ PubMed : 16034288 ]
- Kline S, Rosenberg N. An Overview of Innovation. In: Landau Ralph, Rosenberg Nathan., editors. The Positive Sum Strategy: Harnessing Technology for Economic Growth. 1986.
- Kneller R. The Importance of New Companies for Drug Discovery: Origins of a Decade of New Drugs. Nature Reviews Drug Discovery. 2010; 9 (11):867–882. [ PubMed : 21031002 ]
- Lemley M, Sampat BN. Examiner Characteristics and Patent Office Outcomes. Forthcoming. Review of Economics and Statistics. 2011
- Lichtenberg F. Are the Benefits of New Drugs Worth Their Cost? Health Affairs. 2001;(20):241–251. [ PubMed : 11558710 ]
- Mansfield E. Academic Research and Industrial Innovation: An Update of Empirical Findings. Research Policy. 1998; 7–8 (26):773–776.
- Manton K, Gu X, Lowrimore G, Ullian A, Tolley H. NIH Funding Trajectories and Their Correlations with Us Health Dynamics from 1950 to 2004. Proceedings National Academy of Science USA. 2009; 106 (27):10981–10986. [ PMC free article : PMC2700155 ] [ PubMed : 19549852 ]
- McKeown T. The Role of Medicine: Dream, Mirage, or Nemesis? London: Nuffield Provincial Hospitals Trust; 1976.
- Morlacchi P, Nelson RR. How Medical Practice Evolves: The Case of the Left Ventricular Assist Device. Research Policy. 2011; 40 (4):511–525.
- Moses H III, Dorsey ER, Matheson DHM, Thier SO. Financial Anatomy of Biomedical Research. Journal of the American Medical Association. 2005; 294 (11):1333–1342. [ PubMed : 16174691 ]
- Mowery D, Nelson RR, Sampat BN, Ziedonis AA. Ivory Tower and Industrial Innovation: University–Industry Technology Transfer Before and After Bayh-Dole. Stanford, CA: Stanford University Press; 2004.
- Murphy KM. Measuring the Gains from Medical Research: An Economic Approach. 1. University of Chicago Press; 2003.
- Murphy K, Topel R, editors. Measuring the Gains from Medical Research: An Economic Approach. Chicago, IL: University of Chicago Press; 2003.
- Mushkin S. Biomedical Research: Costs and Benefits. Cambridge, MA: Ballinger Publishing; 1979.
- National Institutes of Health. Cost savings resulting from NIH research support: a periodic evaluation of the cost-benefits of biomedical research. 1993.
- Nelson RR. The Simple Economics of Basic Scientific Research. Journal of Political Economy. 1959;(67):297–306.
- Nelson RR. The Role of Knowledge in R and D Efficiency. The Quarterly Journal of Economics. 1982; 97 (3):453–470.
- Nordhaus. The Health of Nations: The Contribution of Improved Health to Living Standards. In: Murphy Kevin, Topel Robert., editors. Measuring the Gains from Medical Research: An Economic Approach. 2003.
- Philipson T, Jena AB. Who Benefits from New Medical Technologies? Estimates of Consumer and Producer Surpluses for HIV/AIDS Drugs. Forum for Health Economics and Policy. 2006; 9 (2) Biomedical Research and the Economy), Article 3.
- Rosenberg N. Schumpeter and the Endogeneity of Technology: Some American Perspectives. London: Psychology Press; 2000.
- Sampat BN. Academic Patents and Access to Medicines in Developing Countries. American Journal of Public Health. 2009; 99 (1):9–17. [ PMC free article : PMC2636619 ] [ PubMed : 19008514 ]
- Sampat BN. When Do Patent Applicants Search for Prior Art? Journal of Law and Economics. 2010; 53 :399–416.
- Sampat BN. The Allocation of NIH Funds Across Diseases and the Political Economy of Mission-Oriented Biomedical Research, Working paper. 2011.
- Sampat BN, Lichtenberg F. What Are The Respective Roles Of The Public And Private Sectors In Pharmaceutical Innovation? Health Affairs. 2011; 30 (2):332–339. [ PubMed : 21289355 ]
- Scherer FM. The pharmaceutical industry, Chapter 25. In: Culyer Anthony, Newhouse Joseph., editors. Handbook of Health Economics. Amsterdam: North Holland; 2000.
- Sporn MB. The War on Cancer: A Review. Journal of the New York Academy of Sciences. 1997; 833 (1)
- Stevens AJ, Jensen JJ, Wyller K, Kilgore PC, Chatterjee S, Rohrbaugh ML. The Role of Public-Sector Research in the Discovery of Drugs and Vaccines. The New England Journal of Medicine. 2011; 364 (6):535–541. [ PubMed : 21306239 ]
- Stokes D. Pasteur’s Quadrant: Basic Science and Technological Innovation. Washingon Brookings Institution Press. 1997
- Toole AA. Does Public Scientific Research Complement Private Investment in Research and Development in the Pharmaceutical Industry? The Journal of Law and Economics. 2007; 50 (1):81–104.
- Ward MR, Dranove D. The Vertical Chain of Research and Development in the Pharmaceutical Industry. Economic Inquiry. 1995; 33 :70–87.
- Weisbrod BA. Economics and Medical Research. AEI Press; 1983.
- Weisbrod BA. The health care quadrilemma: an essay on technological, change, insurance, quality, and cost containment. Journal of Economic Literature. 1991:523–552.
- Zhang Y, Soumerai S. Do Newer Prescription Drugs Pay for Themselves? A Reassessment of the Evidence. Health Affairs. 2007; 26 (3):880–886. [ PubMed : 17485770 ]
- Zucker L, Brewer M, Darby M. Intellectual Human Capital and The Birth of U.S. Biotechnology Enterprises. American Economic Review. 1998; 88 (1):290–306.
I thank Pierre Azoulay, and participants in the National Academies’ 2011 Workshop on Measuring the Impacts of Federal Investments in Research, for useful comments and suggestions.
Stokes (1997) and others have challenged this definition of “basic” research.
Stokes (1997) provides a thoughtful critique of conventional distinctions between “basic” and “applied” research. Since much of the literature before and since Stokes uses this terminology, we employ it in our review of this literature, even while recognizing the importance of his argument.
Cutler (1998) observes “Common wisdom suggests that rapid cost increases are necessarily bad. This view, however, is incorrect. Cost increases are justified if things that they buy (increases in health) are worth the price paid.” (2)
See however, Zhang and Sourmerai (2007) for a critique of this finding.
The cost-effectiveness of these technologies also depends on the populations on which they are used, as Chandra and Skinner (2011) emphasize.
There is also some discussion about whether the public sector should be paying attention to the cost-side consequences of its investment decisions. Weisbrod (1991) notes: “With respect to the NIH, it would be useful to learn more about the way the size and allocation of the scientific research budget are influenced, perhaps quite indirectly, by the health insurance system, through its impact on the eventual market for new technologies of various types” (535).
Cutler (2008) also emphasizes progress in the “war on cancer” – though highlights the role of screening and personal behavior changes, and notes the high costs of treatment. Sporn (2006) and Balilar and Gonik (1997) offer less sanguine assessments, emphasizing that progress against cancer has been highly uneven. Long-standing debates in assessments of the War on Cancer include the disagreements on the relative importance of treatment versus prevention, and of basic versus applied research. The literature also suggests it is difficult to evaluate the extent of progress in cancer, for two main reasons. First, advances in screening increase incidence. The second is competing risks: for example, the reduction in mortality from cardiovascular disease, discussed above, increased cancer cases. See Cutler (2008) for a review.
A National Cancer Institute (NCI) “Fact Sheet” asserts that “approximately one half of the chemotherapeutic drugs currently used by oncologists for cancer treatment were discovered and/or developed at NCI.” http://www .cancer.gov /cancertopics/factsheet /NCI/drugdiscovery
However, recall that the Toole (2007) study shows that basic research funding by the public sector has a stronger effect on private R and D than clinical research funding.
Heidenreich and McClellan (2003) summarize this point of view in the introduction to their study (discussed above), noting that while previous analyses “have generally not provided direct evidence of the impact on health of specific research studies, or on the likely value of additional research funding” these previous studies tend to conclude “recent gains in health are extraordinarily valuable in comparison with the relatively modest past funding.”
- Cite this Page National Academies (US) Committee on Measuring Economic and Other Returns on Federal Research Investments. Measuring the Impacts of Federal Investments in Research: A Workshop Summary. Washington (DC): National Academies Press (US); 2011. APPENDIX D, THE IMPACT OF PUBLICLY FUNDED BIOMEDICAL AND HEALTH RESEARCH: A REVIEW.
- PDF version of this title (1.6M)
In this Page
- INTRODUCTION AND BACKGROUND
- PUBLIC SECTOR RESEARCH AND OUTCOMES: AN OVERVIEW
- THE EFFECT OF PUBLICLY FUNDED RESEARCH: A REVIEW OF THE EVIDENCE
- MEASUREMENT AND EVALUATION ISSUES
- CONCLUSIONS
Other titles in this collection
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- THE IMPACT OF PUBLICLY FUNDED BIOMEDICAL AND HEALTH RESEARCH: A REVIEW - Measuri... THE IMPACT OF PUBLICLY FUNDED BIOMEDICAL AND HEALTH RESEARCH: A REVIEW - Measuring the Impacts of Federal Investments in Research
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

May Is Celiac Awareness Month
Following a gluten-free diet can relieve celiac disease symptoms and heal damage in the small intestine.
Learn more »

Join Us for Healthy Vision Month 2024!
NEI is shining a light on vision loss and mental health — and sharing steps people with a visual impairment can take to thrive in their daily lives.

RECOVER Initiative launches clinical trials evaluating treatments for exercise intolerance, post-exertional malaise, and sleep disturbances.

Celebrating at graduation this year?
Talk with your high school grads about celebrating safely.

STEM Teaching Resources
Innovative NIH-funded content to engage K-12 students in health science.
In the News

High-Risk Prostate Cancer
New urine test can identify aggressive cancers that need treatment

Parents Lost to Overdose
From 2011-21, more than 321,000 children lost a parent to a drug overdose.

Alzheimer’s Disease
People with two copies the APOE4 gene are at much higher risk.

Fentanyl Pill Seizures
Law enforcement sees massive jump in confiscated amount of the drug.
NIH at a Glance
Virtual-tour-screenshot-square.jpg.

Take the Virtual Tour
Explore the Bethesda campus and how NIH turns discovery into health.

monica-bertagnolli-thumbnail.jpg

The NIH Director
Monica M. Bertagnolli, M.D., is the NIH Director and provides leadership for the 27 Institutes and Centers that make up NIH.
nih-at-a-glance-funding.jpg
Funding for Research
NIH is the largest source of funding for medical research in the world, creating hundreds of thousands of high-quality jobs.
nih-at-a-glance-labs.jpg

Labs at NIH
Scientists conduct research on NIH campuses across the U.S., as part of our Intramural Research Program.
improving-health-collage.jpg

Impact of NIH Research
NIH-supported research has had a major positive impact on nearly all of our lives.
researcher-holding-petri-dish.jpg

Jobs at NIH
The central recruitment point of access to all NIH jobs and training opportunities
A public-private partnership to develop a coordinated research strategy to speed the most promising COVID-19 vaccines and treatments.
Rapid Acceleration of Diagnostics (RADx)
An initiative to speed innovation in the development, commercialization, and implementation of technologies for COVID-19 testing.
A new research initiative to understand, prevent, and treat the long-term effects of COVID-19.
COVID-19 Treatment Guidelines
Guidelines from NIH for the diagnosis, treatment, and control of COVID-19.
Research information from NIH
NIH supports research in COVID-19 testing, treatments, and vaccines. También disponible en español.
NIH COVID-19 Safety Plan
Guidance to NIH staff, including employees, contractors, trainees, and volunteers, related to COVID-19.
Featured Resources & Initiatives
A new science agency proposed by President Joseph Biden as part of NIH to drive biomedical breakthroughs and provide transformative solutions for all patients.
Anti-Sexual Harassment
NIH does not tolerate pervasive or severe harassment of any kind, including sexual harassment.
Ending Structural Racism
Learn more about NIH’s efforts to end structural racism in biomedical research through the UNITE initiative.
All of Us Research Program
A research effort to revolutionize how we improve health and treat disease.
NIH HEAL Initiative
Trans-agency effort to speed scientific solutions to stem the national opioid crisis.
Clinical Trials
Learn about participating in clinical trials and where to find them.
Accelerating Medicines Partnership
A bold venture to help identify new treatments and cures for diseases.
Medical Research Initiatives
Important initiatives aimed at improving medical research.
Training at NIH
NIH provides training opportunities internally, as well as at universities and other institutions across the U.S.
Connect with Us
- More Social Media from NIH
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Americans broadly favor government funding for medical and science research
Americans are strongly supportive of the government investing in research in medicine and science, according to a new Pew Research Center survey.
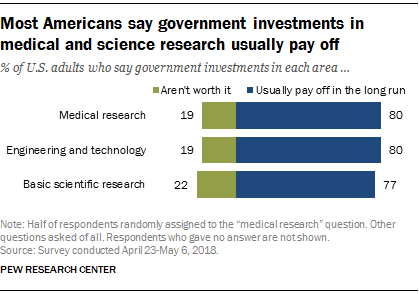
Around eight-in-ten U.S. adults say government investments in medical research (80%), engineering and technology (80%) or basic scientific research (77%) usually pay off in the long run. Only about two-in-ten believe government funding in each of these areas is not worth it (19% for medical research, 19% for engineering and technology and 22% for basic scientific research).
Pew Research Center surveys in 2014 and 2009 also found broad public support for government investments in basic scientific research and engineering and technology, though those surveys used different polling methods and somewhat different question wording. (Views on funding for medical research were not included in the past surveys.)
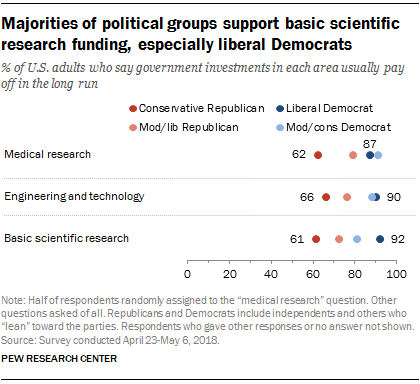
Majorities across the political spectrum agree that government investments in medical research, engineering and technology or basic scientific research pay off in the long run, although there are political differences on these questions. For instance, 92% of liberal Democrats say government investments in basic scientific research ultimately pay off. By comparison, 61% of conservative Republicans say government investments in basic research pay off, while a sizable minority (38%) says government investments in basic scientific research “aren’t worth it.”
A 2017 Pew Research Center survey found a wide and growing political divide over increasing federal spending on scientific research. Democrats and Democratic-leaning independents were 27 percentage points more likely than Republicans and Republican leaners to say they would increase spending on scientific research. In 2001, by contrast, there was no significant divide between Republicans and Democrats over increasing federal spending for scientific research.
The new survey also asked Americans to weigh the importance of government versus private investment in research funding. A majority of Americans (57%) say government funding is essential for scientific progress, while 42% say private funding will ensure enough progress even without government funding. The share saying government investment is essential is broadly consistent with previous Pew Research Center surveys that again used different survey methods and somewhat different question wording.
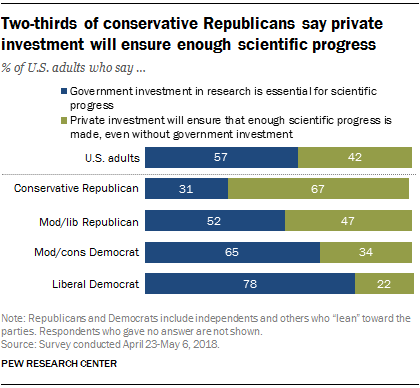
Liberal Democrats and conservative Republicans have different views on this question. About eight-in-ten liberal Democrats (78%) say government funding of research is essential for scientific progress. By contrast, 31% of conservative Republicans say government investment is essential, while two-thirds (67%) say private investment will ensure enough progress is made, even without government funding.
Note: See topline results and methodology here .
- Federal Government
- Health Policy
- Medicine & Health
- Science Funding & Policy

Brian Kennedy is a senior researcher focusing on science and society research at Pew Research Center .
Americans rate their federal, state and local governments less positively than a few years ago
Nearly three-quarters of americans say it would be ‘too risky’ to give presidents more power, the changing face of america’s veteran population, americans’ dismal views of the nation’s politics, what the data says about food stamps in the u.s., most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
Opportunities & announcements, funding strategy for grants, grant writing & approval process, managing grants, clinical research, small business research.
The National Institute of Mental Health (NIMH) is the largest funder of research on mental disorders in the world. Below you can find NIMH funding opportunities and announcements, including those specific to clinical research and training. Also, learn more about NIMH funding strategies, the application process, and grants management.
Notify the NIMH Press Team about NIMH-funded research that has been submitted to a journal for publication, and we may be able to promote the findings.

Australian Government Department of Health and Aged Care
Medical Research Future Fund
The Medical Research Future Fund (MRFF) is a $22 billion long-term investment supporting Australian health and medical research. The MRFF aims to transform health and medical research and innovation to improve lives, build the economy and contribute to health system sustainability.
Learn about the MRFF
Find out what the MRFF is, how its funding works, who manages it and how it is helping Australians to have a healthier future.
See all awarded MRFF grants
Discover comprehensive information about the projects the MRFF is funding in this searchable spreadsheet.
View our grants calendar
See which grants are open, when applications close, and when we expect to award funding.
Apply for MRFF grants
The government's grants hub – GrantConnect – handles all MRFF grant applications.
Latest news

$10 million for research to address areas of unmet health need using existing data infrastructure in new ways

Helping the Northern Rivers community to heal after the 2022 floods

Transforming health and medical research in Australia
Mrff projects.

Improving cancer outcomes by helping regional patients join clinical trials

Smart glasses could help people who are blind or vision impaired navigate by sound

Measuring the wellbeing of First Nations youth

Medical Research Future Fund 2nd 10-year Investment Plan (2022–23 to 2031–32)
The $6.3 billion MRFF 2nd 10-year Investment Plan outlines the Australian Government’s plans for the use of MRFF funding from 2022–23 to 2031–32. It updates and builds on the first 10-year Investment Plan.
Read the report

All MRFF initiatives
Learn about the range of research initiatives we’re funding to improve the health of Australians.

MRFF reports
Read the latest reports from the Medical Research Future Fund.

MRFF videos and webinars
Hear from prominent members of the research community talking about how the MRFF is helping Australians. Learn about the MRFF through our webinars.

Subscribe to MRFF updates
Subscribe to our newsletter to keep you up to date with MRFF news.
Latest resources

Medical Research Future Fund Research Administration Officer Webinar presentation – 27 March 2024

Medical Research Future Fund Research Administration Officer Webinar – 27 March 2024

Improving alignment and coordination between the Medical Research Future Fund and NHMRC’s Medical Research Endowment Account – Consultation

Medical Research Future Fund webinar: How to improve the health of rural, remote and regional communities with an MRFF grant – 14 March 2024
- Health data and medical research
Is there anything wrong with this page?
Help us improve health.gov.au
If you would like a response please use the enquiries form instead.

- Policy & Compliance
- Clinical Trials
Clinical Trial-Specific Funding Opportunities
All applicants proposing clinical trials must submit applications through a notice of funding opportunity (NOFO) that is specifically designated for clinical trials.
For due dates on or after January 25, 2018, NIH requires all applications involving one or more clinical trials to be submitted through a notice of funding opportunity specifically designated for clinical trials. The purpose of this policy is to improve our ability to identify proposed clinical trials, ensure that key pieces of trial-specific information are submitted with each application, and uniformly apply trial-specific review criteria.
Policy Implementation
Applications and proposals involving clinical trials with due dates on or after January 25, 2018 must be submitted to a notice of funding opportunity (NOFO) or request for proposal (RFP) that explicitly states it will accept clinical trials. Since January 25, 2018, NIH no longer accepts clinical trial applications through previous parent announcements. Remember that NIH supports many types of clinical trials (mechanistic, exploratory/developmental, pilot/feasibility, pragmatic, behavioral, and others), so be sure to read your funding opportunity carefully for specific instructions and considerations. It is also recommended that applicants check the online version of their funding opportunity within 8 weeks before the due date to ensure it is still appropriate for their application.
Check Which Opportunities Allow Clinical Trials
To confirm whether a NOFO allows clinical trials, check the opportunity notice's Title and Section II. Award Information for the following designations:
The NIH Guide includes a filter option to Search for Clinical Trial Funding Opportunities .
Notices of funding opportunities that accept clinical trials have specific review criteria to ensure that reviewers appropriately consider clinical trial-related information.
Special Considerations
Basic experimental studies with humans (besh).
These studies fall within the NIH definition of a clinical trial and also meet the definition of basic research .
- All notices of funding opportunities for Basic Experimental Studies with Humans (BESH) are designated as "Required -– Basic Experimental Studies with Humans: Only accepting applications that propose clinical trial(s) that also meet the definition of basic research" in Section II. Award Information.
- These notices of funding opportunities are designated as "Basic Experimental Studies with Humans Required" in the funding opportunity title.
- Participation in notices of funding opportunities for Basic Experimental Studies with Humans will vary by NIH Institute and Center (IC). Many ICs will continue to accept Basic Experimental Studies with Humans through existing notices of funding opportunities that accept clinical trials. However, BESH studies responsive to NOFOs other than designated BESH NOFOs will not have registration and results reporting flexibilities temporarily afforded to those responsive to the designated BESH NOFOs. It is important to check with a Program Officer to determine the most appropriate notice of funding opportunity for your application.
- Funding Opportunity Types by Clinical Trial Allowability
- Basic Experimental Studies Involving Humans (BESH)
Career Development (K) Awards
Career Development awards may support either independent clinical trials or a mentored research training experience , depending on the funding opportunity.
- Notices of funding opportunities that indicate "Independent Clinical Trial Required” in the title and in Section II. Award Information support independent clinical trials conducted by the applicant.
- Career Development applicants proposing to gain mentored training in a clinical trial are instructed to provide details of their contribution to the study in the Research Strategy rather than in the clinical trial specific fields on the PHS Human Subjects and Clinical Trials Information form.
- NIH expects the mentor or individual receiving support for the larger trial to have the overall responsibility of the trial.
Fellowships (F) Awards
The NIH encourages fellows to receive training in clinical research; however, NIH supported fellows are not permitted to conduct a clinical trial independently.
- Fellowship (F) notices of funding opportunities do not include this designation in the funding opportunity title.
- Fellowship applicants proposing to gain mentored training in a clinical trial are instructed to provide details of their contribution to the study in the Research Strategy rather than in the clinical trial specific fields on the PHS Human Subjects and Clinical Trials Information form.
- NIH expects the mentor or individual receiving support for the clinical trial to assume overall responsibility of the trial.
Training (T) Awards
Institutional Training awards do not support clinical trials (with the exception of some D43 and K12 awards).
- Training (T) notices of funding opportunities do not include this designation in the funding opportunity title.
- D43 and K12 notices of funding opportunities are designated as "Clinical Trial Not Allowed" or “Clinical Trial Optional” in the funding opportunity title and Section II. Award Information. These applicants will only be permitted to complete fields for Delayed Onset Studies in the PHS Human Subjects and Clinical Trials Information form.
- Refer to the Comparison of NOFO Types by Clinical Trial Allowability for help determining which notices of funding opportunity to use.
- Check the Annotated Funding Opportunity for help navigating NIH notices of funding opportunities.
- Watch the How to Choose the Right NOFO for more information.

- Updates to Funding Opportunity Terminology
- Continued Extension of Certain Flexibilities for Prospective Basic Experimental Studies with Human Participants
- Notice of Intent to Publish Parent Funding Opportunities for Basic Experimental Studies with Humans
- Delayed Enforcement and Short-Term Flexibilities for Some Requirements Affecting Prospective Basic Science Studies Involving Human Participants
- Reminder: Policy on Funding Opportunities for Clinical Trials Takes Effect January 25, 2018
- Guide Notice on Clinical Trial Funding Opportunity Policy
- NIH Plans for Clinical Trial Specific Parent R01 and Parent R21 Funding Opportunities
This page last updated on: March 26, 2024
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health

IMAGES
VIDEO
COMMENTS
Grants & Funding. The National Institutes of Health is the largest public funder of biomedical research in the world. In fiscal year 2022, NIH invested most of its $45 billion appropriations in research seeking to enhance life, and to reduce illness and disability. NIH-funded research has led to breakthroughs and new treatments helping people ...
Funded Research (RePORT) Access reports, data, and analyses of NIH research activities, including information on NIH expenditures and the results of NIH-supported research. ... Grants.gov ; USA.gov - Government Made Easy ; National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892;
AHRQ contract opportunities and requests for proposals, including information on SAM.gov, important notices and the Contract Solicitation Archive. Policies and procedures, grant announcements, contract solicitations, special initiatives, call for partners, small business innovation research, and research dissertations, training, and career ...
* President Biden proposed the creation of the Advanced Research Projects Agency for Health (ARPA-H) to improve the U.S. government's ability to speed biomedical and health research. Public Law 117-103 was enacted on March 15, 2022, authorizing the establishment of ARPA-H within the U.S. Department of Health and Human Services.
From 2016-2020, industry increased funding for medical and health R&D by 42.8%, federal government by 53.5%, academic and research institutes by 19.6%, and state governments by 5.9%. Foundation investments in medical and health R&D, though down from earlier funding highs in 2016 and 2017
In the last 10 years, the federal government has increased funding for research and development (R&D)—investing $179.5 billion in FY 2021. DOD and the Department of Health and Human Services received 77% of the FY 2021 funding. COVID-19 stimulus funding led to large R&D increases for HHS. For example, an HHS agency that helps develop vaccines ...
September 8, 2021 - By Amrika Ramjewan, Principal Strategist - Mayo Clinic Innovation Exchange. Each year, U.S. government agencies with extramural research and development (R&D) budgets invest in federal funding programs designed to stimulate technological innovation and foster entrepreneurial activity. Coordinated by the U.S. Small ...
For the 10 largest funders, health research funding totalled to $ 37.1 billion, approximately 40% of all spending on health research globally by public and philanthropic sources [].The United States National Institutes of Health (NIH) contributed the largest part of this amount, with $ 26.1 billion in health research funding in 2013.
federal research funding, but carefully consider how to simultaneously ensure private sector R&D investment ... voluntary health associations and professional societies and 23.9% for state and local government. • Overall, U.S. medical and health R&D grew by $51 billion or 35.7% from 2013 to 2018, yet still accounts for just five cents of ...
Health. Measuring the health returns to publicly funded medical research has been a topic of interest to policymakers for decades. In an early influential study, Comroe and Dripps (1976) consider what types of research (basic or clinical) are more important to the advance of clinical practice and health. The authors rely on interviews and expert opinion to determine the top ten clinical ...
Official website of the National Institutes of Health (NIH). NIH is one of the world's foremost medical research centers. An agency of the U.S. Department of Health and Human Services, the NIH is the Federal focal point for health and medical research. The NIH website offers health information for the public, scientists, researchers, medical professionals, patients, educators,
Around eight-in-ten U.S. adults say government investments in medical research (80%), engineering and technology (80%) or basic scientific research (77%) usually pay off in the long run. Only about two-in-ten believe government funding in each of these areas is not worth it (19% for medical research, 19% for engineering and technology and 22% ...
Funding. The National Institute of Mental Health (NIMH) is the largest funder of research on mental disorders in the world. Below you can find NIMH funding opportunities and announcements, including those specific to clinical research and training. Also, learn more about NIMH funding strategies, the application process, and grants management.
Both government and industry research funding increased rapidly from between the years of 1994-2003; industry saw a compound average annual growth rate of 8.1% a year and slowed only slightly to a compound average annual growth rate of 5.8% from 2003 to 2008. ... and the top 50 institutions received 58% of NIH medical research funding, the ...
Institutional Eligibility. In general, domestic or foreign, public or private, non-profit or for-profit organizations are eligible to receive NIH grants. NIH may limit eligibility for certain types of programs, such as limitations on the participation of foreign entities or programs for which only small businesses are eligible applicants.
The Medical Research Future Fund (MRFF) is a $22 billion long-term investment supporting Australian health and medical research. The MRFF aims to transform health and medical research and innovation to improve lives, build the economy and contribute to health system sustainability. ... The government's grants hub - GrantConnect - handles ...
For due dates on or after January 25, 2018, NIH requires all applications involving one or more clinical trials to be submitted through a notice of funding opportunity specifically designated for clinical trials. The purpose of this policy is to improve our ability to identify proposed clinical trials, ensure that key pieces of trial-specific ...